Is there any phenomena where the wave description of electron (motion) is not applicable?
Let us touch base and have a look at this bubble chamber picture:
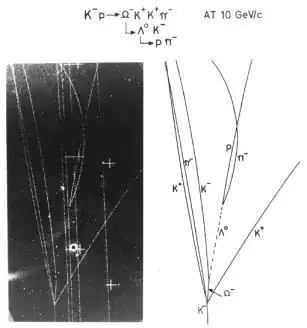
This is one single elementary particle interaction, rare, because it is the creation of an omega particle, but it is one single instant.
There is nothing wavelike in this datum. The particles behave in the magnetic field exactly like charged classical particles are expected to behave and their momentum and mass can be measured or fitted to great accuracy.
This is a particle interaction. The point I want to make is that even though this is a reaction that can only be mathematically modeled statistically by quantum mechanics, there is absolutely no manifestation of the wave nature of elementary particles in this one instant.
We might have a single electron turning in the field . It is not interesting enough to be recorded for teaching purposes, but again it would not display any wave properties.
In conclusion, in the quantum mechanical framework, single events/instances can be described by classical trajectories and physics. It is when the statistics are accumulated that the wave behavior appears. The statistical distribution of such scatterings will be a probability distribution given by the quantum mechanical wave equations, and will display the wave nature of the underlying framework. The waves in quantum mechanics are probability waves . Many instances must be accumulated in a distribution to manifest the wave nature . In the double slit experiment with single electrons a single electron does not express any wave nature. One can calculate its trajectory classically after the fact. One cannot predict the trajectory unless the probability wave nature of the underlying framework is taken into account.
To summarize for individual measurements the wave nature may not appear at all or cannot be predictive of a trajectory. What the wave nature does is predict a statistical distribution for the particles under consideration. In the example above, a statistical distribution will give the crossection for producing omega- in kaon interactions, angular distributions etc , and these distributions can only be explained with the wave nature of quantum mechanical entities.
The reason for this question is really to find out if there are any situations were quantum wave theories fail.
The quantum wave theory is continually validated by all experiments. If there were a failure it would have meant a major disturbance in the physics community. Everything is consistent with the particle manifestation for individual events, probability wave distributions for statistical accumulations.
If not it seems that the Everett's multiworld analysis as described by Coleman, http://media.physics.harvard.edu/video/index.php?id=SidneyColeman_QMIYF.flv works.
This last is not clear, seems to me a non sequitur .
