Consider a closed composite system with an internal movable adiabatic wall. If we release the piston, thermodynamics cannot determine the final equilibrium state (the temperature cannot be determined).
(About the previous example, you can refer to Callen's Thermodynamics and an Introduction to Thermostatistics 2ed. P53 Problem 2.7-3 and P100 Problem 4.3-1:
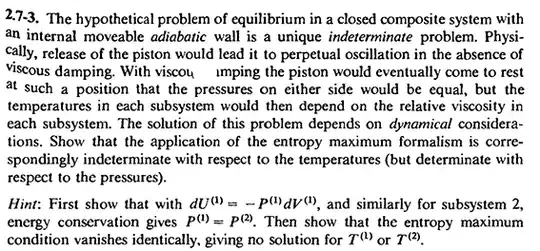 )
)
How to understand this? Consider a quasi-static process. As the piston moves, the temperature and the pressure of both chambers will change until the pressures coincide. Then there is a well-defined unique final equilibrium state. What is wrong with this argument?
Does it mean that thermodynamics is incomplete?