When thinking about small particles and their uncertainty, I've always rather seen them being all over the place rather than randomly changing location. I would think that, in the same time, you'd always have same result, have you tried to do something to them (eg. shoot another particle at them).
You have to define "particle". When going to dimensions below a nanometer the term "particle" is not the classical particle as a billiard ball. Depending on the experiment/observation it will give an answer "particle" or "wave". This does not mean that it is spread out all over the place. It only means that using quantum mechanical solutions for the problems under consideration we do not get trajectories or orbits, but just probabilities of how probable it is to find the electron ( as an example) at ( x,y,z,t). This means that to get any reliable value we have to accumulate statistics.
The best way is to contemplate the two slit experiment with single electrons. In this experiment you throw an electron against two narrow slits with small separation .
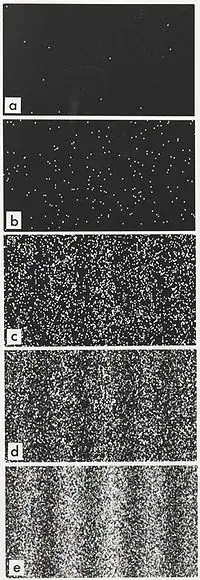
The single dots in the upper picture are single electrons which have crossed the two slits and then interacted with the screen as a dot, a given ( x,y,z). After statistical of many throws, an accumulation gives the probability distribution ( which is the square of the quantum mechanical solution for the problem, the state function), and we see the wave nature appearing. Also an uncertainty of position on average ( which would allow us to know the momentum with great accuracy)
This makes me wonder: if you try something with uncertain particles, does every experiment end up differently because, event though all conditions are the same? (The last requirement seems to be unfeasible so consider it just a thought experiment).
As you see in the experiment above, yes, each single outcome has an uncertainty. It is the accumulated distribution that gives the numbers to compare with predictions.
As for the Big Bang, our observations lead us to the observable universe and we have a model for it. In the accepted theoretical model there is only one BB.
Out of the question, I must say that I find it very confusing for particles to really have random locations. Wasn't there ever a suspicion that their location (as well as the probability of it) has a rule we can find out?
Hidden variable theories have problems with the Lorenz invariance which is something that all of our experimental observations say holds without exception.
