I have learned so many concepts under astrophysics and unfortunately, I have muddled everything together... Let me try to illustrate my problem:
When a star is in main sequence, it fuses hydrogen to produce helium and energy that is mostly given off as light (This is what I learned from watching a few youtube videos)
Also, a star is a black body and has a spectrum that looks like this:
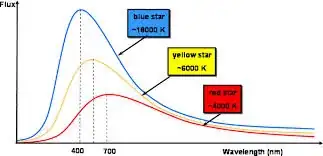
Furthermore, if I split the light that comes from the sun, I get an emission spectrum of a few colors which correspond to the colors absorbed by the elements on the sun's surface
To wrap up what I am confused about...
Does the process go like this...
During the fusion of hydrogen, energy is given off as EM waves
The amount of the different sorts of EM waves is shown by the curve above
As the EM waves move up the surface, it is absorbed by the elements on the surface and then re-radiated... this is the emission spectrum
I am having trouble linking up the different concepts together and articles on the net are complicating things further... could someone please tell me which part of my concepts is wrong?