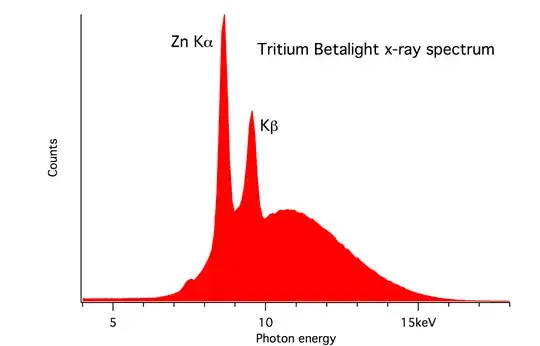
After consultation with Pieter who has the most authoritative answer, I will throw in my scintillator results. This image is the result of scanning a tritium keychain with a Hamamatsu R6095 PMT and a 25x10mm NaI crystal running at 800V.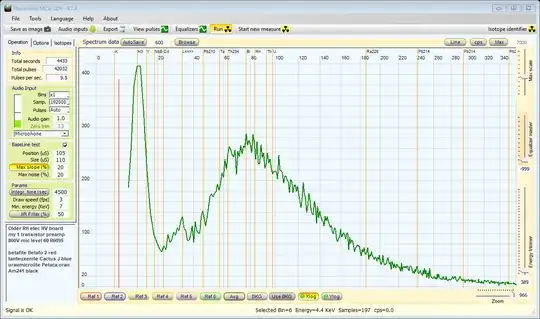 Based on Pieter's Bremsstrahlung diagram I have placed the peak at 11keV. The rest of the graph is background radiation.
Based on Pieter's Bremsstrahlung diagram I have placed the peak at 11keV. The rest of the graph is background radiation.
So the answer is that the "usual gamma detector" will indeed detect the Bremsstrahlung that results from the tritium beta decay. By "usual" I mean of course that you are using a high enough voltage on your scintillator so that the energies above at least 10keV are visible.
And, no, it is not caused by weak secondary X-Ray radiation generated when electrons travel through the keychain's plastic. It is caused by Bremsstrahlung xrays as the original beta particle passes close to positively charged nuclei. Diverting the electron from its original path causes a loss in kinetic energy that results in the emission of a photon.