 | |
 | |
| Names | |
|---|---|
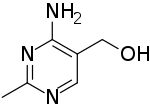
| Preferred IUPAC name
(4-Amino-2-methylpyrimidin-5-yl)methanol | |
| Other names
Pyramin[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.234.283 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H9N3O | |
| Molar mass | 139.158 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Toxopyrimidine is a vitamin B6 antagonist with potent convulsant effects.[2][3]
See also
References
- ↑ Haughton BG, King HK (December 1958). "Toxo-pyrimidine phosphate as an inhibitor of bacterial enzyme systems that require pyridoxal phosphate". The Biochemical Journal. 70 (4): 660–5. doi:10.1042/bj0700660. PMC 1196724. PMID 13607425.
- ↑ Rindi G, Ferrari G (February 1959). "The gamma-aminobutyric acid and glutamic acid content of brains of rats treated with toxopyrimidine". Nature. 183 (4661): 608–9. Bibcode:1959Natur.183..608R. doi:10.1038/183608a0. PMID 13632808. S2CID 4200644.
- ↑ Rindi G, Perri V, Ventura U (April 1959). "Effect of toxopyrimidine on glutamic-decarboxylase and glutamic-oxalacetic transaminase of rat brain". Nature. 183 (4668): 1126–7. Bibcode:1959Natur.183.1126R. doi:10.1038/1831126a0. PMID 13657025. S2CID 4209134.
External links
 Media related to Toxopyrimidine at Wikimedia Commons
Media related to Toxopyrimidine at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.