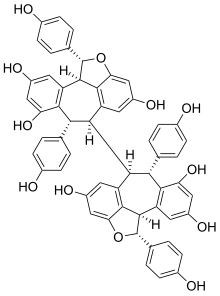
Chemical structure of hopeaphenol.
Oligostilbenoids (oligo- or polystilbenes) are oligomeric forms of stilbenoids. Some molecules are large enough to be considered polyphenols and constitute a class of tannins.[1]
Examples
Dimers
Trimers
- α-Viniferin
- Ampelopsin E[2]
- trans-Diptoindonesin B
- Gnetin H[4]
Tetramers
- cajyphenol A[3]
- cajyphenol B[3]
- Flexuosol A[5]
- Hemsleyanol D[2]
- Hopeaphenol[4]
- Vaticanol B[2]
- R2-Viniferin (syn. Vitisin A)
Modified
- Diptoindonesin C can be isolated from the bark of Shorea pinanga[4]
Other
- Diptoindonesin F can be isolated from the bark of Shorea gibbosa[2]
Glycosides
References
- ↑ Boralle, N; Gottlieb, H.E; Gottlieb, O.R; Kubitzki, K; Lopes, L.M.X; Yoshida, M; Young, M.C.M; Oligostilbenoids (1993). "Gnetum venosum". Phytochemistry. 34 (5): 1403–1407. doi:10.1016/0031-9422(91)80038-3. INIST 4012160.
- 1 2 3 4 5 Saroyobudiono, Haryoto; Juliawaty, Lia D.; Syah, Yana M.; Achmad, Sjamsul A.; Hakim, Euis H.; Latip, Jalifah; Said, Ikram M. (2008). "Oligostilbenoids from Shorea gibbosa and their cytotoxic properties against P-388 cells". Journal of Natural Medicines. 62 (2): 195–198. doi:10.1007/s11418-007-0205-0.
- 1 2 3 4 Bao, Li; Ma, Xiaofeng; Song, Xiaohong; Wang, Manyuan; Liu, Hongwei (2010). "Two New Resveratrol Tetramers Isolated from Cayratia japonica (Thunb.) Gagn. With Strong Inhibitory Activity on Fatty Acid Synthase and Antioxidant Activity". Chemistry & Biodiversity. 7 (12): 2931–2940. doi:10.1002/cbdv.200900394. PMID 21162006.
- 1 2 3 Yana M. Syah; Euis H. Hakim; Emilio L. Ghisalberti; Afghani Jayuska; Didin Mujahidin; Sjamsul A. Achmad (2009). "A modified oligostilbenoid, diptoindonesin C, from Shorea pinanga Scheff". Natural Product Research. 23 (7): 591–594. doi:10.1080/14786410600761235. PMID 19401910.
- ↑ Li, Wen-wu; Li, Bo-Gang; Chen, Yao-zu (1998). "Flexuosol A, a New Tetrastilbene fromVitis flexuosa". Journal of Natural Products. 61 (5): 646–7. doi:10.1021/np970457v. PMID 9599267.
External links
 Media related to Oligostilbenoids at Wikimedia Commons
Media related to Oligostilbenoids at Wikimedia Commons
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.