 | |
| Names | |
|---|---|
| Preferred IUPAC name
N1-(3-Aminopropyl)propane-1,3-diamine | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 1071254 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.238 |
| EC Number |
|
| 26839 | |
| KEGG | |
| MeSH | norspermidine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2269 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H17N3 | |
| Molar mass | 131.223 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Ichtyal, ammoniacal |
| Density | 938 mg mL−1 |
| Melting point | −16 to 0 °C; 3 to 32 °F; 257 to 273 K |
| Boiling point | 240.60 °C; 465.08 °F; 513.75 K |
| log P | −0.826 |
Refractive index (nD) |
1.481–1.482 |
| Hazards | |
| GHS labelling: | |
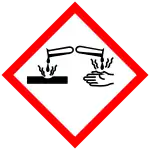  | |
| Danger | |
| H302, H311, H314, H317, H330 | |
| P260, P280, P284, P305+P351+P338, P310 | |
| Flash point | 117 °C (243 °F; 390 K) |
| 280 °C (536 °F; 553 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
738 mg kg−1 (oral, rat) |
| Safety data sheet (SDS) | fishersci.com |
| Related compounds | |
Related amines |
|
Related compounds |
Agmatine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Norspermidine is a polyamine of similar structure to the more common spermidine. While norspermidine has been found to occur naturally in some species of plants,[1][2] bacteria,[3] and algae,[4] it is not known to exist in humans.
Norspermidine is being researched for use as a cancer medication.[5][6]
References
- ↑ Rodriguez-Garay, B; et al. (1989). "Detection of Norspermidine and Norspermine in Medicago sativa L. (Alfalfa)". Plant Physiology. 89 (2): 525–529. doi:10.1104/pp.89.2.525. ISSN 0032-0889. PMC 1055875. PMID 16666576.
- ↑ Hamana, K; et al. (1998). "Unusual polyamines in aquatic plants: the occurrence of homospermidine, norspermidine, thermospermine, norspermine, aminopropylhomospermidine, bis(aminopropyl)ethanediamine, and methylspermidine". Can. J. Bot. 76 (1): 130–133. doi:10.1139/cjb-76-1-130.
- ↑ Yamamoto, S; et al. (Apr 27, 1979). "Occurrence of norspermidine in some species of genera Vibrio and Beneckea". Biochem Biophys Res Commun. 87 (4): 1102–1108. doi:10.1016/S0006-291X(79)80021-2. PMID 313792.
- ↑ Hamana, K; Matsuzaki, S (1982). "Widespread Occurrence of Norspermidine and Norspermine in Eukaryotic Algae". J. Biochem. 91 (4): 1321–1328. ISSN 0021-924X. PMID 7096289.
- ↑ Prakash, NJ; et al. (1988). "Antitumor activity of norspermidine, a structural homologue of the natural polyamine spermidine". Anticancer Res. 8 (4): 563–568. PMID 3140710.
- ↑ Sunkara, PS; et al. (1988). "Mechanism of antitumor activity of norspermidine, a structural homologue of spermidine". Adv Exp Med Biol. 250: 707–716. doi:10.1007/978-1-4684-5637-0_62. PMID 3255245.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.