 | |
 | |
| Names | |
|---|---|
| Other names
sodium dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.038.834 |
| EC Number |
|
| E number | E331i (antioxidants, ...) |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
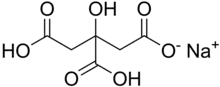
| C6H7NaO7 | |
| Molar mass | 214.105 g·mol−1 |
| Appearance | white powder hygroscopic |
| Odor | odorless |
| Melting point | 212 °C (414 °F; 485 K) |
| Boiling point | 309.6 °C (589.3 °F; 582.8 K) |
| soluble | |
| Solubility | negligible in ethanol |
| Acidity (pKa) | 3.50–3.80 |
| Structure[1] | |
| Monoclinic | |
| P21/a (No. 4) | |
Formula units (Z) |
4 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
5400 mg/kg (mouse, oral) >2000 mg/kg (rat, dermal) |
| Safety data sheet (SDS) | Carl Roth |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Monosodium citrate, more correctly, sodium dihydrogen citrate (Latin: natrium citricum acidulatum), is an acid salt of citric acid. Disodium citrate and trisodium citrate are also known. It can be prepared by partial neutralisation of citric acid[2] with an aqueous solution of sodium bicarbonate or carbonate. It has a slightly acidic taste.[2]
- NaHCO3 + C6H8O7 → NaC6H7O7 + CO2 + H2O
- Na2CO3 + 2C6H8O7 → 2NaC6H7O7 + CO2 + H2O
It is highly soluble in water and practically insoluble in ethanol.[2] Monosodium citrate is used as an anticoagulant in donated blood.[3] It is used as an alkalinizing agent to prevent kidney stone disease.[4] The crystals form as nearly perfect cubes.[5]
References
- ↑ Glusker, Jenny P.; van der Helm, D.; Love, Warner E.; Dornberg, Marilyn L.; Patterson, A. L. (June 1960). "The State of Ionization of Crystalline Sodium Dihydrogen Citrate1". Journal of the American Chemical Society. 82 (11): 2964–2965. doi:10.1021/ja01496a071. ISSN 0002-7863. Retrieved 22 July 2022.
- 1 2 3 "Monosodium Citrate - Jungbunzlauer". www.jungbunzlauer.com. Retrieved 17 July 2022.
- ↑ Clinical Hematology: Theory and Procedures, Mary Louise Turgeon
- ↑ PubChem. "Monosodium citrate". pubchem.ncbi.nlm.nih.gov. Retrieved 2021-08-02.
- ↑ Hitchcock, David I. (March 1946). "Sodium Hydrogen Citrates". Journal of the American Chemical Society. 68 (3): 524–525. doi:10.1021/ja01207a507. ISSN 0002-7863. PMID 21015754. Retrieved 22 July 2022.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.