 | |
| Names | |
|---|---|
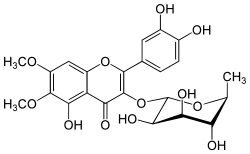
| IUPAC name
3′,4′,5-Trihydroxy-6,7-dimethoxy-3-(α-L-rhamnopyranosyloxy)flavone | |
| Preferred IUPAC name
2-(3,4-Dihydroxyphenyl)-5-hydroxy-6,7-dimethoxy-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
2-(3,4-Dihydroxyphenyl)-5-hydroxy-6,7-dimethoxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H24O12 | |
| Molar mass | 492.433 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Eupatolin is a chemical compound. It is a flavonol rhamnoside attached at the 3 position to an eupatolitin molecule. It can be found in Eupatorium ligustrinum.[1]
References
- ↑ Quijano, L.; Malanco, F.; Ríos, Tirso (1970). "The structures of eupalin and eupatolin. Two new flavonol rhamnosides isolated from Eupatorium ligustrinum D.C". Tetrahedron. 26 (12): 2851–2859. doi:10.1016/S0040-4020(01)92863-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.