 | |
| Names | |
|---|---|
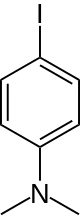
| Preferred IUPAC name
4-Iodo-N,N-dimethylaniline | |
| Other names
N,N-Dimethyl-4-iodoaniline | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H10IN | |
| Molar mass | 247.079 g·mol−1 |
| Appearance | dark blue to purple solid [1] |
| Density | 1.652 g/cm3[2] |
| Boiling point | 263.7 °C (506.7 °F; 536.8 K) |
| 34.64 mg/L | |
| Vapor pressure | 2 mmHg |
| Hazards | |
| GHS labelling: | |
  | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
4-Iodo-N,N-dimethylaniline, usually called 4-iododimethylaniline, is an organic compound with the formula IC6H4N(CH3)2. It is a dark blue to purple solid. The compound is used to attach the dimethylanilinyl group to other substrates.
Synthesis
4-Iodo-dimethylaniline is prepared by iodination of dimethylaniline.[3]
- C6H5NMe2 + I2 → I:C6H4NMe2 + HI
The iodination is so efficient that it has been recommended for quantifying the presence of iodine.[4]
References
- ↑ SDS from LGC Standards https://www.lgcstandards.com/DE/de/4-Iodo-N-N-dimethylaniline/p/TRC-I719595-100MG last checked 24.11.2023
- ↑ ACDLabs. "N,N-Dimethyl-4-iodoaniline". ChemSpider.
- ↑ Boothe, Richard; Dial, Christopher; Conaway, Richard; Pagnl, Richard M.; Kabalka, George W. (1986). "The iodination of aromatic substrates on alumina". Tetrahedron Letters. 27 (20): 2207–10. doi:10.1016/S0040-4039(00)84488-3.
- ↑ Mishra S, Singh V, Jain A, Verma K. K. "Determination of iodide by derivatization to 4-iodo-N,N-dimethylaniline and gas chromatography-mass spectrometry" Analyst. 2000, vol. 125, p. 459-64.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
