Question: given a path taken by a system through state space, is it possible to make a statement such as 'that path corresponds to an irreversible process' or 'that path corresponds to a reversible process'?
Further remarks: I've been thinking about this in the context of ideal gases and $p-V$ diagrams. We often talk about a gas expanding reversibly and isothermally. Is it possible for a gas to expand isothermally and irreversibly? Or if you tried to enact such an expansion, would you not (by virtue of not expanding the gas infinitely slowly) cause small changes in temperature that would cause the path to deviate from a perfect isotherm? If such deviations would occur, then it would appear that the isotherm can be identified as describing a reversible process, with no additional information about how the path is traversed required.
To express it conversely: can, for a given path in state space, we always achieve reversibility by moving along it infinitely slowly? That is to say, is it in fact the case that 'reversibility and irreversibility' are properties which are not at all dependent on path taken, but rather on how the path is traversed as a function of time?
I feel as though I've seen images like this:
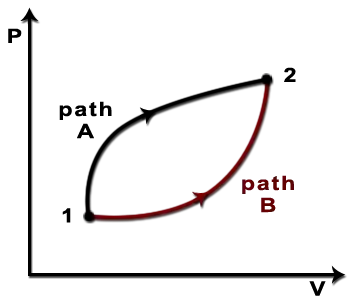 $$ $$
in which the paths are identified as corresponding to an irreversible and a reversible process respectively. This is consistent with another idea: I've often heard it stated that we can write $dW = -p\,dV$ for reversible processes only. This equation I have always found somewhat perplexing, since the left hand side is seemingly an inexact differential, whilst the right hand side seemingly exact! Is the resolution of this that the condition of reversibility restricts us only to certain paths for which the integral of $dW$ is indeed path-independent? Or perhaps $p\,dV$ is not an exact differential, since the variable $p$ is not solely a function of $V$? By this I mean: if $p = p(V,X)$ (where $X$ is just some other variable), then the integral of $p\,dV$ between two points would indeed depend on the path through $(V,X)$ space taken.
$$ $$
in which the paths are identified as corresponding to an irreversible and a reversible process respectively. This is consistent with another idea: I've often heard it stated that we can write $dW = -p\,dV$ for reversible processes only. This equation I have always found somewhat perplexing, since the left hand side is seemingly an inexact differential, whilst the right hand side seemingly exact! Is the resolution of this that the condition of reversibility restricts us only to certain paths for which the integral of $dW$ is indeed path-independent? Or perhaps $p\,dV$ is not an exact differential, since the variable $p$ is not solely a function of $V$? By this I mean: if $p = p(V,X)$ (where $X$ is just some other variable), then the integral of $p\,dV$ between two points would indeed depend on the path through $(V,X)$ space taken.
Yet more remarks: in traditional treatments of thermodynamics, after a discussion of the Carnot cycle and reversible cycles in general, the following result is arrived at:
$$ \oint \frac{dQ_\mathrm{rev}}{T} = 0 \,.$$
Using standard results of multi-variable calculus, this implies that
$$\int_\mathrm{init}^\mathrm{final} \frac{dQ_\mathrm{rev}}{T} $$
is path-independent. But if reversibility is indeed a function of path, what does it mean to integrate $dQ_\mathrm{rev}/T$ along an irreversible path? I've understood $\Delta Q_\mathrm{rev}$ for an arbitrary process to mean 'the heat that would be absorbed by the system between its initial and final states if it were to move between the states by a reversible process'. So does the above integral mean 'integrate $dQ/T$ along a host of adiabats and isotherms (i.e. a zig-zagging reversible path) that best approximates the irreversible path'?
Or is it in fact the case that irreversible processes simply cannot be represented by $p-V$ diagrams, for instance? A gas which is expanded quickly enough as to generate pressure waves would not have a well-defined pressure, and so we would not be able to represent it by a point in state-space, perhaps?
Thank you.