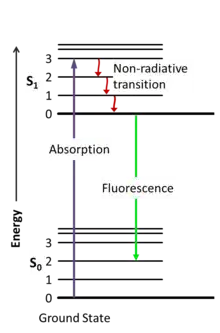
It is often said that substances, objects have color because they selectively absorb all color of sunlight except one. The wavelength that is not absorbed reaches our eyes and we perceive it as "color". This "color" phenomenon is often described as light of a certain wavelength being reflected. I think it is more appropriate to call it scattering. Scattering is one thing and fluorescence is another. Both processes can give a substance "color". I have some "yellow" powder that is fluorescent and its fluorescent color is green....
Scattering, deep down, is not a classical wave phenomena either. It still deals with molecules and atoms absorbing photons and emitting photons....
In essence, what is the difference between scattering (elastic or nonelastic) and fluorescence from an electronic transition point of view?
Best, Kavan