I cannot understand how a $COP > 1$ does not contradict the laws of thermodynamics (the first one, in particular).
Whatever searches I do, return things like "a heat pump is moving heat, not creating it" .... The answer in my mind is: "Yea, cute phrasing, I don't care: as far as I can see, the mechanism violates the first law of thermodynamics".
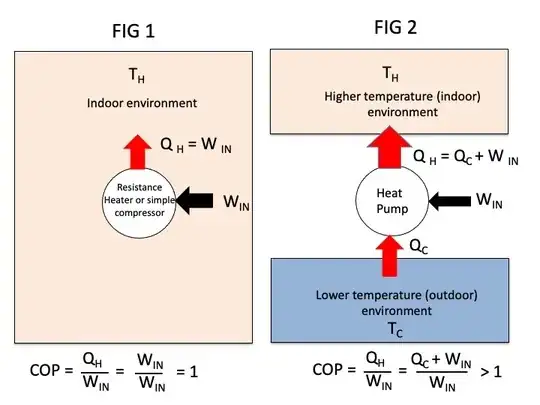
My understanding is that a heat pump is essentially (conceptually) like a fridge, or like an air conditioner, only that it is "cooling down the outside" (which is colder).
Using a simplified mode of operation where the operations are "lumped" or "discrete". Also to simplify, let's assume that the outside and inside temperatures are the same, $T$; the outside is considered to have an infinite amount of air, so its temperature will not change by the operation of our pump.
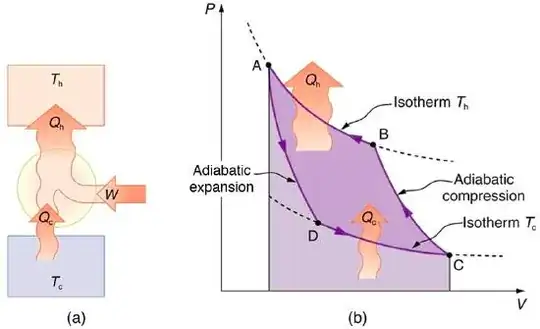
So, in my mind, the thing would go like: take a container with some gas; we mechanically compress it by spending an amount of work $W$. Then:
$(1)$ By the first law of thermodynamics, the amount of heat $Q$ that the gas got is exactly the amount of mechanical work that we applied to compress it; $Q = W$.
So, we now wait until it complete cools down. It cools down to approximately $T$ (the amount of air inside and its heat capacity far exceeds the amount of gas that we compressed).
Now we take it outside and let it expand, which does by doing a mechanical work of exactly $W$, and by the first law of thermodynamics, it will cool down by releasing an amount of heat of exactly $Q$.
We let it warm up and reach temperature $T$.
The cycle now repeats.
We spent exactly $W$ energy, and injected an amount of heat $Q_{IN} = Q = W$. As far as my understanding goes, $Q_{IN}$ cannot be greater than $Q$ and thus greater than $W$.
The simplifications actually work in the pump's favor. If the temperature outside was colder, the efficiency would actually be lower.
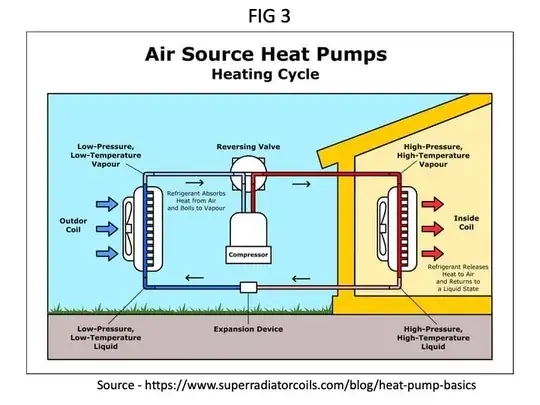
So, what am I missing? (I know that the actual operation is based on a continuous compressor and a valve, with heat exchangers in the path — but my understanding is that this is representative of how that setup works, just easier to visualize — is this assumption mistaken?)