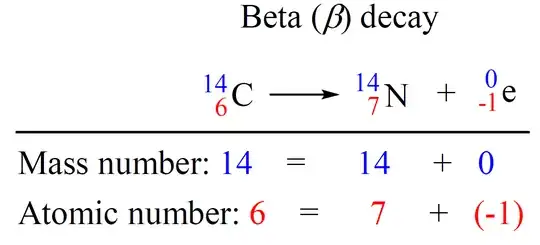
I am confused why beta decay can emit energy. While I know proton decay is impossible because the neutron weighs more than the proton I think we can apply the same logic for other reactions. Consider the reaction:
Carbon 14 mass: 14.003 242 Da
Nitrogen mass: 14.003 074 Da
Electron mass: 0.000 548 Da
Thus the mass of the products is 14.003 622 daltons which is more energy than the reactant and therefore it must absorb energy, right?