I've heard that since sun is around 5800K hot at its surface, the hottest temperature one can get with solar heating is that temperature. From a thermodynamics standpoint, this makes very much sense. An object cannot heat another object to a higher temperature than itself.
However, I am not exactly satisfied with that answer. The sun emits around $3.8\cdot10^{26} W$ of power. If one gathers even a fraction of that and then concentrates that power to a small point, shouldn't it not heat up to millions of degrees or more to balance out the heat coming in with heat radiated out via $\frac{P}{A}=\sigma T^4$?
One explanation I've heard that the albedo of the object itself becomes higher and higher as the temperature approaches the incoming temperature, 5800K in this case. To me, this sounds a bit ridiculous, though. How can be recieving object know the temperature it should not go over, and then act appropriately? It's like the object itself performs some fourier analysis of the incoming radiation, deduces that the radiation is thermal, then calculates the absolute temperature and finally changes its own albedo accordinly. I didn't know rocks or tables could think and alter their own properties.
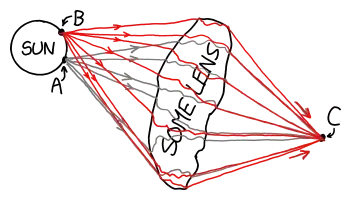
Even if the scenario about the albedo that I presented above would make some sense, I still refuse to believe that if you gather the entire solar output for one second and shoot it at a pencil, that the pencil gets heated up just to 5800K.
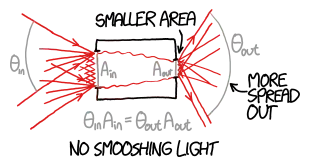
The final thought I had for the example given above, is the realization that the light gets concentrated. If one concentrates the solar light, then the 5800K constraint should be removed right? Well, from a thermodynamics standpoint this still does not make that much sense to me at least. In order for an object (sun) to heat up another object (target) to a higher temperature than itself, additional energy should be supplied. This is how a heat pump works. However, if one uses only mirrors, there is no additional energy anywhere, so this cannot be the full explanation.
Any thoughts on this?