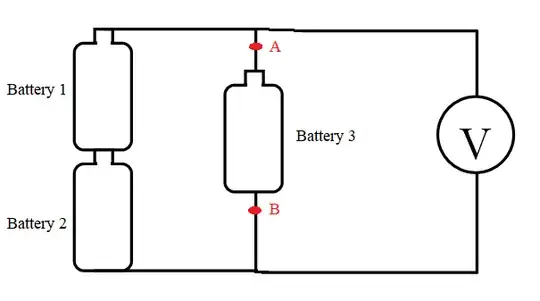
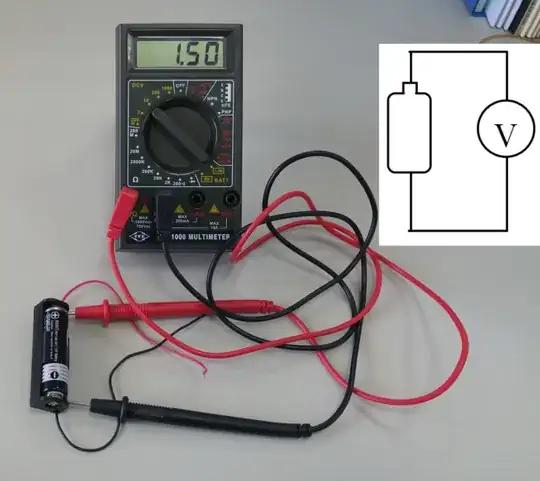
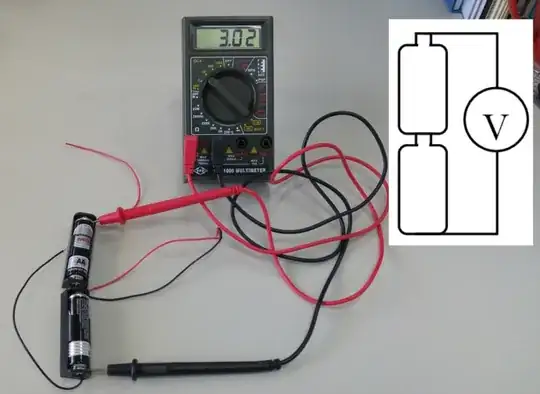
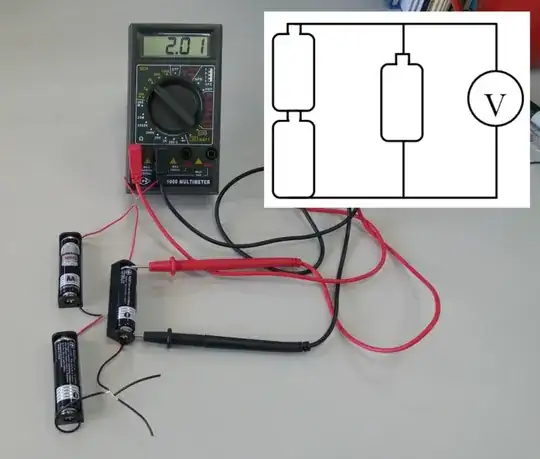
While the other answers dealt with the electrical aspects of the experiment, I will add somewhat of a chemistry touch:
These 1.5V ZnMnO2 cells come in two flavours:
- Alkaline cells. The electrolyte is water solution of potassium hydroxide.
- Salt cells. The electrolyte is a water solution of ammonia chloride.
Both of these chemistries (esp. the salt cells) are PARTIALLY rechargeable. This means that, with some luck, you can recharge a partially discharged cell to somewhat higher state of a charge. On the other hand, the commercially available cells are not designed to be charged, so one could expect neither a high cycle life (my own experiments some 35y ago yielded ~15 cycles between ~30% and ~80%) nor much of a safety.
What can go wrong?
- Gas and pressure buildup. Both types use water-based electrolyte and the parasitic chemical reactions when you attempt to charge such a cell (esp. when the only limit of the current is its own internal resistance) tend to decompose the water and create hydrogen and/or oxygen with a tiny scent of chlorine and ammonia from the salt cells.
Cells are generally made gastight in order to prevent the electrolyte leaks (making the cells safer) and to limit the water vapor loss (improving the shelf life). Cell walls also have a finite tensile strength, so a pressure buildup will lead to a rupture and a disappointing explosion. The explosion will be disappointing because the walls have intentionally weak places - again for improving safety. What you will get is a "Pfffff" type of explosion.
Anyway, the event can be somewhat dangerous to the eyes, the skin or the clothes.
At least wear a safety goggles if you want to see the whole spectacle.
- A lot of heat, leading to a steam pressure buildup in the two discharged cells. See the above for the effects of the increased pressure.
Some higher-quality cells have safety features that will switch off the cell on heat or pressure buildup, thus preventing the Pfffff event.
Other, lower-quality cells may happen not to be this much gastight, so instead of a small explosion you could hear the hissing of the escaping gases.
Some, even lower-quality cells may start a fire.
Your mileage may vary.
The electrolyte that may leak is not immediately dangerous, but still not good when in contact with any part of human body. Wash with water.
Be sure not to repeat the experiment with any type of lithium battery or with any type of battery exceeding 20g in mass.