We measure a particle's size by plastically scattering a point particle off it, e.g., to measure the protons size, we do:
$$ e^- + p \rightarrow e^- + p $$
and look at the cross section versus momentum transferred to the target ($|q|$).
You then compare it to the formula for point particle scattering (Mott scattering)
$$ \sigma = \sigma_{Mott} F(q^2) $$
and get a nice figure:
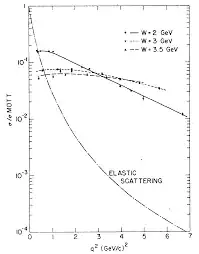
where all we care about is the smooth curve labeled "ELASTIC SCATTERING".
Note that:
$$ \hbar c = 0.2\,{\rm GeV\cdot fm}$$
so the figure here goes to $7\,(GeV/c)^2$m so it probes distances down to:
$$ L = \frac{\sqrt{7\,(GeV/c)^2}}{\hbar c} \approx 10^{-16}\,{\rm m}\approx R_{proton}/10 $$
Moreover, $F(q^2)$ (called the Elastic form factor) is the Fourier transform of the radial charge distribution (which is spherically symmetric for the proton). so elastic scattering measures the charge distribution of the target particle. (There is also one for the magnetic dipole distribution).
For electrons:
$$ \rho(r) = \delta(r) $$
so far. Idk what the current limit is for electrons, but it's much smaller than the proton radius. There is no sign of of structure for any of the quarks or leptons.
Note that pion and photon form factors have been measured. The latter is possible because photons have hadronic content via the $\rho^0$ meson.
