Yes; oxides can be reduced to pure metals in low-oxygen atmospheres, especially with heating.
Recall that Nature acts to reduce the Gibbs free energy $G\equiv H-TS$ (enthalpy $H$, temperature $T$, entropy $S$) for systems in thermal and mechanical contact with their surroundings. A negative $\Delta G=\Delta H-T\Delta S$ corresponds to spontaneity. Note the tradeoff between the enthalpy and entropy changes; either a sufficient enthalpy decrease (from strong bonding) or a sufficient entropy increase (from additional particle configurations) can drive a process forward.
And the higher the temperature, the stronger the influence of $\Delta S$.
From this we can conclude that any reaction we see that turns a gas into condensed matter—which decreases entropy, $\Delta S<0$—must be exothermic ($\Delta H<0$, and indeed $\Delta H<T\Delta S$). Oxidation eliminates gas and thus produces a large entropy decrease. So why is oxidation (sometimes) spontaneous? Because the strong bonding of the compound releases an exothermic latent heat of reaction that heats the universe, increasing its entropy more than enough to compensate.
At higher temperatures, though, the entropy decrease makes the $-T\Delta S$ term too large, and oxidation ceases to be spontaneous; instead, reduction occurs into pure metal as the oxidation reaction reverses.
This is shown graphically by the Ellingham diagram:
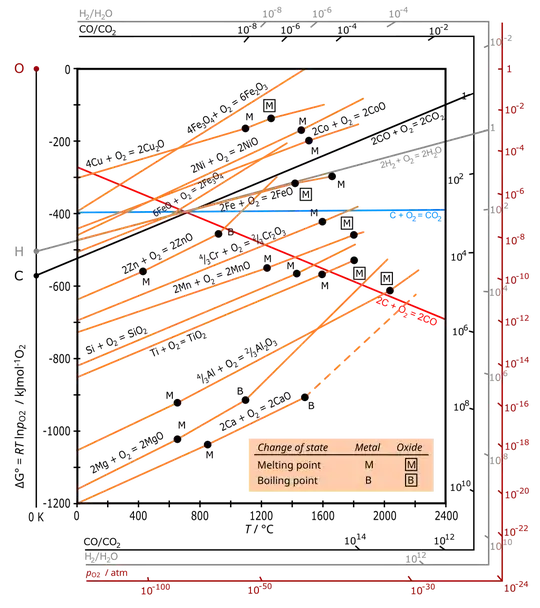
The way to use this diagram is to extend a line from the $\mathrm O$ point on the top left to the reaction of interest at the temperature of interest. The line then passes though the equilibrium partial pressure of oxygen. A higher partial pressure makes oxidation spontaneous; a lower partial pressure makes reduction spontaneous. Note, however, that the partial pressure you need to maintain to reverse rusting at room temperature is very small indeed, and that the diagram shows only equilibrium states, without information on how fast the process occurs.
