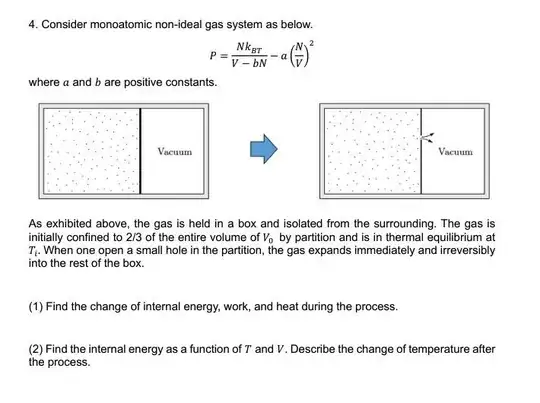
the problem is diffusion of non-ideal gas
i think, in this situationm the change of internal energy, work, and heat during process is zero because this process is free expansion(diffusion)
but, how can i get internal energy as function of T and V? when i tried $$dU = C_v\Delta T$$ or $$dU=C_p\Delta T$$ but the pressure and volume is not constant. how can i approach this??