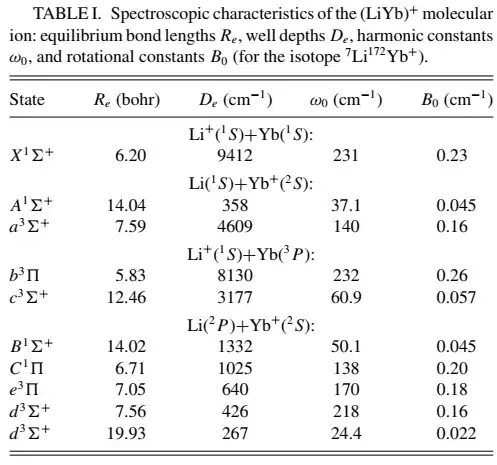
I'm reading about papers about atom-ion interaction. There are some states as showned above. The notations of the states look like molecular orbital since $\Sigma$ and $\Pi$ is usually used in orbital hybridization to denote the different alignments of wavefunctions. But the letters before $\Sigma$ and $\Pi$ and the superscripts are confusing me.
What are the letters (both lower and upper case) and all the superscript (numbers and +)?