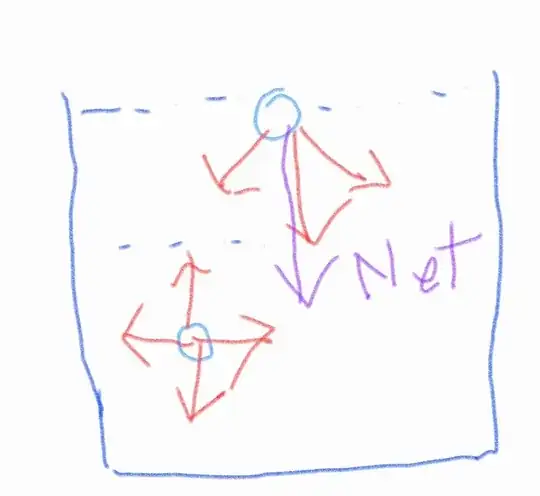
Why doesn't that molecule accelerate and come downwards in that case?
This question is not easy to answer very precisely, because this specific molecule might move around in the liquid, so per se it is not staying where it is. And also the forces you drew are not the "only" forces acting on this molecule, it is also affected by gravity and other molecules hitting it accidentally from below (in large scale this correspond to the water pressure).
According to Newton's 2nd law, since there is a net force acting on the molecule should come downwards as long as the net force keeps acting. Why is there violation of Newton's law in fluid here?
So the thing is in your picture you miss the pressure from below and the gravity. While it might not be true on a molecule level, the liquid (or parts of it) is not accelerating, because these forces cancel each other out. The molecules from below hit the "surface molecule" which pushes it upward, and the gravity and the cohesion pulls it down, cancelling this.
If you want to get one step even deeper in the explanation, there isn't really a point of separating the cohesive force from the "pushing each other" force, since both are simply different parts of a Lennard-Jones like potential (this is a strong simplification, water molecules have directional dependence etc...):
- When the molecules are very close they push each other apart
- When they are further there is an attraction between them
Also, how does the above phenomenon explain surface tension? I read on almost everywhere but still can't get it why the molecular explanation suggests that water surface should behave as an elastic material?
I think this is easier to understand if you approach it from an energetic perspective. If you look at the LJ potential (which is still a huge simplification), it shows that water molecules "like" to be close (not too close) to each other, than being very far. If you look at a molecule in the bulk of water, it has many neighbours (surrounded in all directions), while a molecule on the surface has way less. This means that a molecule in the bulk is energetically more favourable than on the surface. So what water "tries" to do is to minimize its surface, so put as many molecules in the bulk as possible.
This is the reason why in case of no gravity, water droplets form spheres. The sphere is the shape which has the smallest surface, given a specific volume.