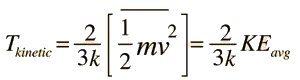If all the particles of a gas had the same speed, the same direction,
did not collide and were not subject to any external force, would the
gas have a temperature,
What you are describing is the kinetic energy of a container of particles as a whole with respect to an external frame of reference, i.e., the macroscopic kinetic energy of the container of gas.
However, in addition to the collective motion and kinetic energy of the gas, there is also always the kinetic energy associated with the random motion of the gas particles with respect to the center of mass of the container of gas. The temperature of the gas is based on the average kinetic energy of the those molecules, not the collective kinetic energy of the container of molecules as a whole.
And what if all the particles had the same speed, were not subject to
an external force but had random directions? Does the gas have a
temperature?
But the random speed of the particles of a gas cannot have the same speed. they follow the Boltzmann distribution. Only the collective motion of the particles can have the same speed, and that collective motion does not influence the temperature of the gas.
If a gas A (10000 particles) had 1000 times more particles than a gas
B (10 particles) and the particles of each gas had the same average
kinetic energy, would the two gases have the same temperature ?
Yes.
But they would not have the same total internal energy. For an ideal gas, where the internal energy consists only of kinetic energy, gas A would have 1000 times more internal energy than gas B.
Hope this helps.