When I tried to find out why UV rays cannot penetrate through glass, I found this answer. But then I thought of gamma rays. Why should gamma-ray bursts or cosmic radiation, be of concern to us then? But gamma rays are of such short wavelengths, they probably won't even penetrate through the atmosphere(according to that answer). But the fact is that we do worry about gamma rays causing mass extinctions, so that must not be the case.
This answer attempted to explain just this, but I find it a contradiction to the previous one.
So am I understanding it wrong? Can you please explain what really goes on on the microscopic scale?
- 1,647
2 Answers
In general, the penetrating power of EM radiation becomes stronger as its wavelength gets shorter. However, this depends on the particular composition of the material it is penetrating- which is why for example ordinary soda-lime window glass will block IR while pure quartz glass will pass it, and why certain wavelengths of sunlight are strongly absorbed by some components of the earth's atmosphere.
We study gamma rays, x-rays and infrared radiation from deep space because those things are tell-tales for certain processes going on in distant stars, gas clouds and galaxies. In the example of gamma-ray bursts, the ones we can detect here have so far been extremely faint because their sources are so very far away from us. Be advised though that if one went off in our neighborhood, the gamma rays would be so intense that enough of them would make it through the atmosphere to kill us.
- 99,024
The energy of UV light 400-200nm is about 3-6 eV (electron volts). Gamma rays if produced by nuclear processes are between several hundred thousand eV and about 8 million eV. The energy spectrum of gamma ray bursts are somewhere between 100 and 5000 keV. Both UV and gamma rays are electromagnetic waves, but the UV absorption is primarily by exciting an electron from a lower energy level to a higher energy level which in a glass or semiconductor usually means from a valance band to a conduction band. In the glass, or insulator we don't usually think of them being conductive, because electrons are not thermally excited to the higher band.
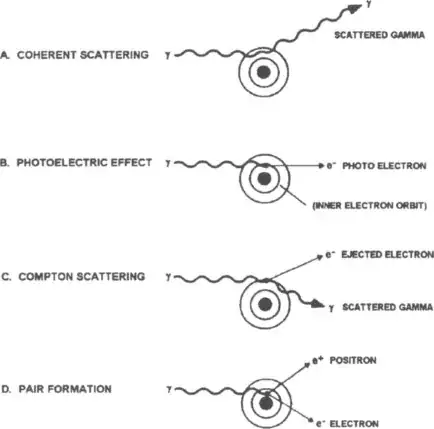
In the case of gamma rays you can have more interactions.
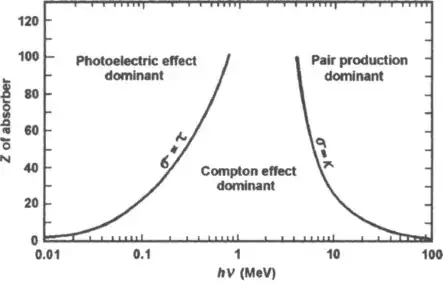
And if you look at the absorption as a function of energy
You can see for the higher energy like from your gamma ray burst is pair production. So in that since UV and Gamma Ray absorption will have different mechanisms. The high energy gamma ray as it interacts will matter will produce an ionization trail. This can kill electronic devices, damage DNA, etc.
The denser the material the more gammas will be absorbed per unit length. This gives an exponential decrease as you increase the thickness of material. The atmosphere is not that dense, but is relatively long. So there will be absorption of the gamma rays. The number that reaches the ground depends on the starting number.
The problem with gamma ray and extinction events is that the intensity so high, that the atmosphere would not stop them all, and a lot of the energy would be deposited into the atmosphere as well as the the rest of the earth.
- 3,338