Background
So in classical mechanics, my understanding is that for the action by using a the principle of least action one can get the equations of motion. Adding a constant to the action does not change anything.
However, the above argument relies on the notion of continuity and thus fails in collisions. For example, if my physical system is a ball seen at $2$ points under no other forces then the principle of least action predicts that the path taken must be a straight line (whereas it may have rebounded of a wall as well)
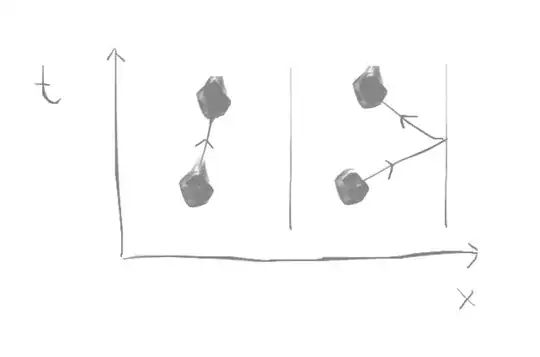 (where $t$ is time and $x$ is the position)
(where $t$ is time and $x$ is the position)
Question
Now, I understand why is it that by adding a constant in the action density always have non-observable consequences if one assumes continuity. My question is how does one prove this when there are discontinuities as well?
My attempt
Consider the Lagrangian $\mathcal{L_M}$ for a gas. Generally in the gas ideal model only the kinetic energies are considered but let us think of the potential energy of a collision and not assume the collision is an event in spacetime but has finite duration. The turning point can be thought as a consequence of regularisation.
The potential experienced by $2$ objects when they collide is given by: $$ V_{exp} = \frac{1}{2} \mu v_{rel}^2 $$
where $V_{exp}$ is the potential experienced, $\mu$ is the reduced mass and $v_{rel}$ is the relative velocity. The new action density when collisions are included is given by:
$$S(p) \to S(p) + S_c$$
where $p$ is the momentum, $S(p)$ is the action when only kinetic energies are considered and $S_c$ is the action contributed by the potential energy. Now, if I assume a short ranged interaction:
$$ S_c = \int L_c dt \approx V_{exp} \tau $$ where $\tau$ is the collision duration. Now for a gas, the number of collisions per $4$ volume is given by:
$$ d N_c = \frac{1}{2}\rho^2 A |\langle v_{rel} \rangle | dt dx dy dz$$ where the $\rho$ is the density, $A$ is the area of the molecule and $dt$, $dx$, $dy$, $dz$ are infinitesimals. Hence, the action density $\tilde S_c$ for the entire gas is given by:
$$ \tilde S_c \approx \frac{1}{4} \rho^2 A |\langle v_{rel} \rangle | \mu\langle v_{rel}^2 \rangle \langle \tau \rangle $$
Now while $\langle \tau \rangle$ should depend on the short range potential I see no reason it couldn't possibly have the term (after using the equation of state):
$$ \langle \tau \rangle = \frac{C}{\rho^2 |\langle v_{rel} \rangle | \langle v^{2}_{rel} \rangle } + \dots $$
where $C$ is a constant?