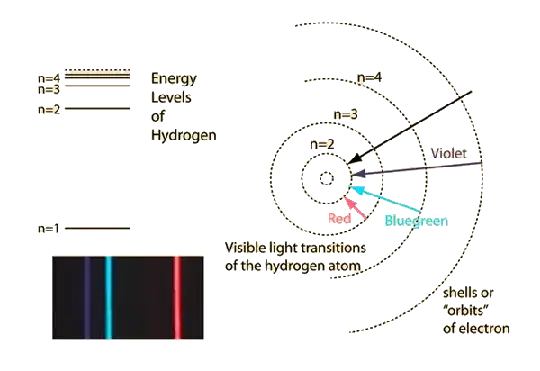
I have a very basic doubt in Bohr's Atomic Model. I just studied that an electron can go to any Energy State with in the atom, by getting relevant energy from photons. If an electron goes to 2nd Energy Level from Ground state,can it come back to 1st Energy State from it,then in ground state?? When I asked my teacher,he said....yes it can But my question is how?... Like, suppose the energy gap between ground and 1st energy level is 6eV. The electron got it somehow from an photon(can it get some no of photons to acquire this energy?,or it will just get 1?),and reaches 2nd energy state. Suppose this photon is a photon of Violet light. I observed in a YouTube video,when it come back from 2nd Energy level to the 1st, it emitted a different color of ray(not violet),then it emitted another color of ray(as photon),but not violet. My question is how it is possible?
Like, I gave it Violet colour photon and it emitted some different colors?
How?
If I eat rice,I can only vomit rice, Not any other kind of food, right?
Please explain me how it happens?
With some simple examples....
Thanks...