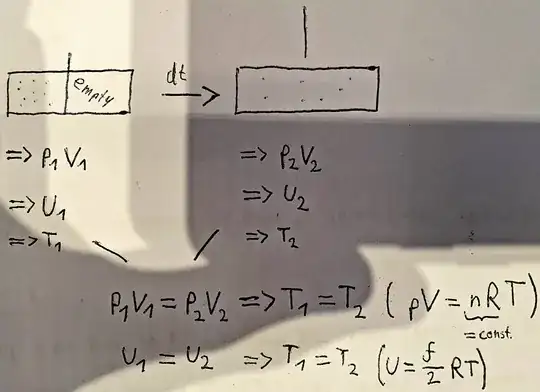
If I were to instantaneously remove the wall as drawn above, then there is no loss of molecular velocities --> T1 = T2.
But I have a problem imagining that there really is no change in temperature. If I put a thermometer in the system with the wall still inside, wouldn't there be significantly more molecules bumping the thermometer in a period of time than in the larger volume because it is more concentrated there? Or would I have to imagine a thermometer that somehow covers the entire system?
I imagine that as more time passes between two impacts of a molecule with the thermometer (which must be the case with larger volumes) the measured temperature is lower.
I know that the temperature must remain the same, as nothing changes in the mean kinetic energy, but I would like to have my error of reasoning found, please, can someone tell me what is wrong?
(I strongly suspect that my thought is wrong, that the thermometer "cools down" again in the time between impacts, that mercury actually has no possibility to transfer heat when there is nothing there (there is nothing to transfer between two impacts), in this case I have the following question: Suppose I had a body and would transfer a lot of energy to it in the form of heat, thereby its temperature rises strongly to T0, if I now instantaneously convey it into a perfect evacuated space, would T0 remain constant?)