In the wiki article on the electronic band structure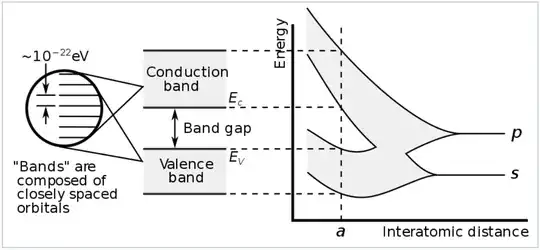 illustrates on how the valence and conduction bands of group 4 semiconductors like diamond emerge from the 2s and 2p orbitals of the C atoms.
illustrates on how the valence and conduction bands of group 4 semiconductors like diamond emerge from the 2s and 2p orbitals of the C atoms.
Chemistry textbooks as far as I know state that individual C atoms create sp3 hybrid orbitals BEFORE forming bonds to other atoms. If one assumes only a single sp3 orbital widening to a band upon interaction between many atoms, then in the logic of the figure only one band will form without the possibility for a band gap.
Is there a reason why the figure displays the interaction of explicitly 2s and 2p orbitals leading to the band structure? Or are the valence/conduction bands for e.g. diamond rather a result of the interaction between many bonding/anti-bonding bonding/anti-bonding states formed by sp3-sp3 bonds as this question suggests? Is there someone around with firmer knowledge than myself to comment on my puzzlement?