As far as I've seen, E&M waves are sinusoidal. Our eyes observe these patterns and give us colors, but in a situation like such: Why do electrons emit radiation? There is still a wave, or at least a pulse, but it's not necessarily sinusoidal, and I'm not sure what 'wavelength' would be used. Nonetheless, radiation produced in this way does have a wavelength-it is used in synchrotrons. So what am I missing?
Asked
Active
Viewed 64 times
1 Answers
1
When electromagnetic radiation comes in pulses, as in synchrotron radiation, the pulse contains many frequencies because the angle of emission defines the radiation, and the angle is not unique, so there is a spread in frequencies.
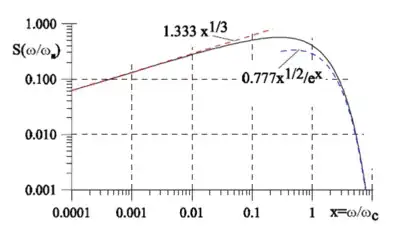
Frequency distribution of radiated energy
given in the plot as a ratio on the critical frequency. The calculations are not simple but the plot shows the frequency spread in a synchrotron pulse.
anna v
- 236,935