These are exactly the same question I struggled with when I was young, but answers are rather simple.
- What happens with the two electrons:
Actually, way more than only 2 electrons get involved.
Think about the energy scale of the alpha decay (few MeV) and the energy scale of the binding between the outer electrons and the nucleus (few eV). A single alpha particle can kick millions of electrons out of their home atoms before it slows enough to think about own electrons. This is how alpha particles are detected - by the vast amount of ions they create.
The nucleus that emits the alpha particle gets enough recoil not only to lose a great deal of its own electrons, but to leave a small ionized trace of its own.
The energy scale is linked to the timescale as well. Electrons regroup around the new nuclei (the alpha particle included) much, much later.
- How the alpha particle manages to escape the atom?
See the energy scale again. If it happens to interact with an electron of the parent atom, the only possible outcome is that the electron is also kicked out, not slowing the alpha particle much.
- Why only four nucleons?
Well, not always. Different isotopes are known to emit electrons or positrons (see beta decay), neutrons (see the neutron drip line), protons (see the proton drip line), alpha particles (you already know) as well as heavier nuclei all the way to splitting the original nucleus roughly in half (see nuclear fission).
Which one of these mechanisms will happen depends mainly on the total energy (mass) of the decaying nucleus and the possible decay products.
Alpha particle is rather easy to emit because it has rather high binding energy, i.e. it has much lower energy than its constituent nucleons when separated.
Sometimes, more than one decay mode is possible when considering the energies involved. They really do happen (at different rates). In the quantum world, if a process is at all possible, it is obligatory.
With a particle accelerator (and patience, and proper detectors) one can kick whatever particle wants from a nucleus - including, but not limited to, deutrons, He-3 and likes.
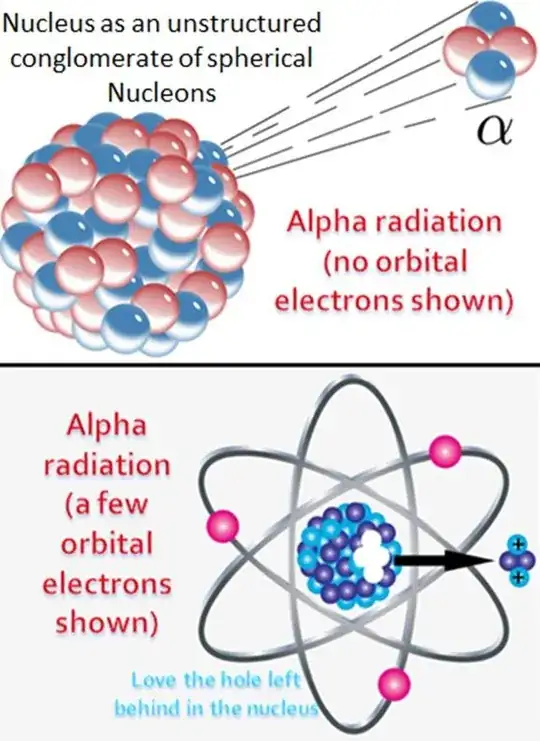
- Unstructured conglomerate of nucleons
Plain wrong approach. Nucleus is pretty much structured - protons and neutrons (separately) occupy orbitals pretty much like the electrons do. They are even called s,p,d,f just like electron orbitals are.
Nucleons are spherical... in a sense. The quarks inside have their own orbitals. Likely s-type because in the nucleons we have at most 2 quarks of the same sort.