I'm learning about such things as ionic and covalent bonds, and the reason given for the ionic bonds is electrostatic attraction. However, if that were true, then the two ions would accelerate toward each other and shortly collide with each other. There must be a force opposite the electrostatic force that is repelling the ions, with exactly the same magnitude so as to cancel out the electrostatic force. (Edit: I'm actually referring to the ionic bond between cations and anions in this first paragraph, like sodium and chloride ions. Below is what I found by researching this topic, dealing mainly with covalent/molecular bonding.)
The following quote is from The Mechanical Universe:
For example, the potential energy of a pair of Hydrogen atoms has a position of stable equilibrium. The potential energies of interaction between atoms are due to the electrical forces between them, causing attraction when they're far apart. At smaller distances, they resist being squeezed together. The result is an equilibrium position where attraction and repulsion are in perfect balance. At that position, they bind in a Hydrogen molecule.
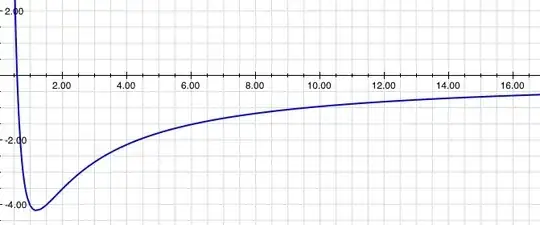
I understand that on a larger (planetary) scale, there is a centrifugal potential that is inversely proportional to the distance $r$ that adds up with the gravitational potential to create this graph:
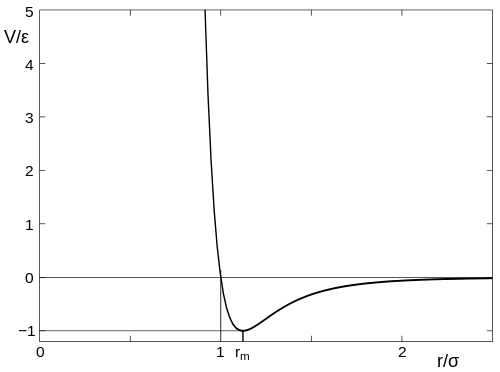
I'm looking for an analogue to this on the molecular scale. I've recently found out about the Lennard-Jones Potential, which gives this equation and curve:$$V_{LJ}=\epsilon \left[ \left(\frac{r_m}{r}\right)^{12} - 2\left(\frac{r_m}{r}\right)^6 \right]$$
In the wikipedia article, it says
...the repulsive term describes Pauli repulsion at short ranges due to overlapping electron orbitals
Later on it is noted that
The repulsive term has no theoretical justification.
So then this is my question: What is the nature of the "Pauli repulsive force"?
If it's possible, I would appreciate an answer with cited sources.
Edit: After some more research, I've found that attraction between two hydrogen atoms (which are both neutral) comes from Induced Dipole-Induced Dipole interaction AKA London Dispersion Forces. Collectively, the attraction and repulsive forces at the molecular scale are dubbed the Van der Waals forces. The wiki on Van der Waals forces mentions A repulsive component resulting from the Pauli exclusion principle that prevents the collapse of molecules. I would like to learn more about how the Pauli exclusion principle manifests itself as a repulsive force between any two atoms/ions.