So I wanted to know that will it won't move if the metal have different potentials in different region of its surface. and what about electrons present in the region of high potential and what about those in lower potential ?
Your question iss within classical electrodynamics, whereas the electrons atoms and molecules belong to the quantum electrodynamics regime. One has to build quantum mechanical models that take into account that even though for single atoms electrons stay at specific energy levels defined by the potential between electrons and nucleus, the collective lattice composed of metal atoms has again a quantum solution that is approximated with quantum models.
In the band theory for solids
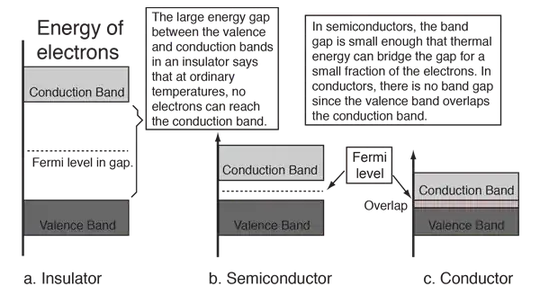
In terms of the band theory of solids, metals are unique as good conductors of electricity. This can be seen to be a result of their valence electrons being essentially free. In the band theory, this is depicted as an overlap of the valence band and the conduction band so that at least a fraction of the valence electrons can move through the material.
The electrons present in lattices of materials are called free electrons. The word ‘free’ refers to, ‘being unbound’. The electrons are free of the atomic boundaries but they are bonded to the surrounding atoms by attractive forces called the metallic bond.
Free electrons, being free to move in row formations, as long as they are being replaced, ‘not removed’ are the reason for electrical conductance among metals.
The metallic bond takes care of the potentials you are worrying about.
