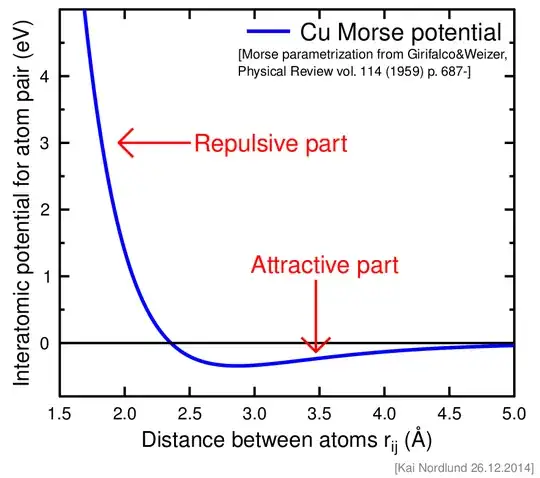
According to the perspective of a high school student,this question has been asked so pardon me for any lack of intuition or knowledge about this topic.
As far as I know as per Coulomb's law or Newton's law, attraction force or repulsion force should decrease as the distance increases between two particles(charged). When a spring is expanded, shouldn't its attraction force decrease as interparticular distance is increasing gradually? Then why does restoring force increase? I know this is an universal truth but I want to know why does this happen