Let's have a look at the thermal-camera image from the company's website, and have a conversation about how cameras work.

The first thing to note is that $\rm 62\,ºF = 17\,ºC$ is a pretty reasonable temperature for an office building or warehouse with very aggressive air-conditioning or with feeble heat in the wintertime. That's almost certainly the temperature of the wall behind the model. It is unlikely to also be the temperature of the spark shower.
Here's a question: how big are these sparks? In the visible-light photo on the left, most of them are hand-sized or longer: call it $\rm 10\,cm$ for a nice round number. But that is clearly not the physical size of the physical sparks, which are zirconium-titanium granules. The company says a $\rm 200\,g$ package runs the machine for ten minutes, so the granules are leaving the device a one gram every three seconds. That $\rm200\,g$ package has a powder volume of about $\rm 40\,mL$. If you don't have a good intuition for this, go into your kitchen, use a big spoon to measure out $\rm 40\,mL$ of sugar, and then try to pour it out over ten minutes. (For extra credit, do your slow pour onto a flame; the sugar crystals will ignite and spark.)
The reason the sparks are long in the visible-light photograph is that they are moving. A photograph doesn't capture an "instant," though that's frequently a useful approximation; a photograph captures an average of all the light that comes into the camera the entire time the shutter is open. (Or, for an all-digital camera, the entire time the CCD sensor is active.) If we make a reasonable guess about the height of the spark shower in the demo, physics tells us how fast the sparks are going; I figure the visible-light image was an exposure between 1/30 and 1/60 of a second.
The sparks show up in the visible-light image, even though they are moving, because they are bright. But if they were dimmer, or if they were moving faster, their light would be spread across more of the image and become harder to distinguish from the background. For an extreme example, consider the image below:

This is a ten-minute exposure of a busy city street, taken by Daguerre in 1838 (source). However, only one person on the street stood still enough for long enough to for his image to stand out from the background. (He seems to be having his boots shined.) Everyone else on the street has disappeared into the noise in the image of the stationary buildings and trees.
In general, an infrared sensor is (a) slower and (b) blurrier than a visible-light camera sensor. The total mass of burning Zr-Ti is, at any instant, probably about 100 milligrams. This is two million times less mass than the human model in the image, with body temperature around $\rm 37\,ºC$.
A thermal-imaging camera that is designed and calibrated for slow-moving solid objects like people or steaks or car engines, with one of those objects actually in its image, might very well show a stream of high-temperature particles as a faint bright streak against the background.
An amusing homework problem would be to make reasonable guesses about the total surface area of the burning granules and the infrared passband for the thermal imager, and try to predict the relative blackbody brightnesses of the spark shower and the human model's arm. Blackbody physics says that the hotter object is brighter in all wavelength bands per unit surface area, but nearly all of the volume of the spark shower is empty space.
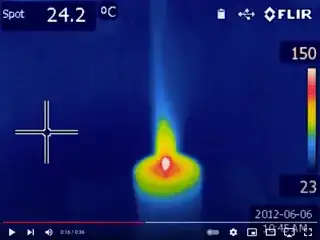

For a similar example, compare this thermal image of a candle (source) to a visible-light image of a candle (source). The brightest visible part of the flame is above the wick, where soot particles are emitting blackbody radiation. But the brightest infrared part of the flame is the hot part of the solid wick (which you sometimes see glowing blackbody-red), followed by the area of the wax pool that is hot enough to vaporize. The visibly-bright part of the flame is a distant third in the thermal image, partially because the flame is "optically thin": you can't see through a flame because it's bright, but it doesn't really cast a shadow.
The fact that this company would design a burning-metal spark machine, point a thermal imaging camera through the spark shower at the wall, and not notice that they can change the temperature of their spark shower by adjusting their thermostat — that's an argument for regulation.





