In my book, it is written that when an atomic gas or vapour is excited either by heating or electrifying it the electrons get excited and immediately de excite and emit light of specific wavelengths. This is how an emission line spectrum is obtained. An emission line spectrum can be considered a fingerprint for identification of a particular element. But I have a question. When we excite the atomic gas or vapour, the electrons in it get excited and when de-excite by jumping down to a lower energy level they emit energy in the form of electromagnetic radiations. But I also studied that when an electron emits a photon by jumping down to a lower energy level it can emit the photon in any random direction. Show all the atoms in the atomic gas or vapour will emit photons in random directions. Thus if we put a screen behind the atomic gas or vapour we won't observe any emission line spectrum because the photons are emitted in random directions, right?
2 Answers
It is correct that atoms will emit photons in random direction. However, some of these photons will be emitted in the direction of the screen, and this will be enough. If, for simplicity, we assume that the vapor fills a cubic volume, and one of the walls of this volume is the screen, then one atom out of 6 will be emitting in the direction of the screen. Note also that we are talking about a macroscopic number of atoms, i.e., the number of the order of the Avogadro constant, $N_A\approx 10^{23}$.
The direction of a photon has nothing to do with its energy. Regardless of direction, a given photon has a well-defined energy that is analogous to its colour. A spectrometer, e.g. a glass prism, can tell you what energies of photon are boiling off the atomic gas by looking at the photons that happen to hit the spectrometer.
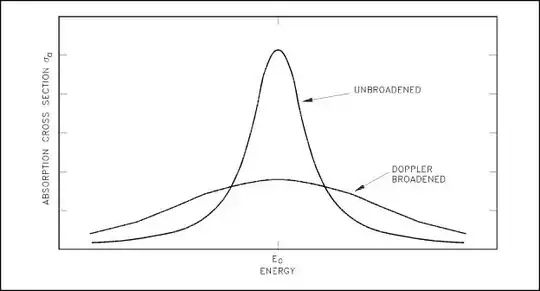
The only sense in which direction matters is for a phenomenon known as Doppler broadening. At finite temperature, the atoms are moving around at random - some will move away from the detector, others towards. When you plot light intensity against frequency, this broadens the peak you get:
- 2,861