I'll try to add something to the excellent answers already posed, mainly in the work of Vieira and Regitano-D'arce about it. I'm afraid this is a chemistry problem, but I don't see a natural division of competence between chemical problems and physical ones, so I will answer anyway.
Despite many opinions to the contrary, I think you are actually right, there is no way to understand that the heating of canola oil in the microwave oven can be explained by a possible polarity of the fatty acid molecules.
Canola oil, food-grade version derived from hexane extraction of apolar substances of rapeseed cultivars specifically bred for low erucic acid content, however, is a very complex and particular mixture.
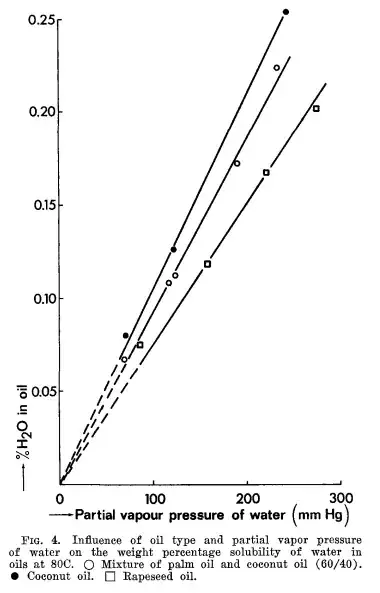
Almost certainly you have some water solved in the canola oil. The idea that oil and water don't mix is just a qualitative idea, they always mix a little. Hilder (1998) estimates about 1% water content in edible oil. That's what is sometimes called pyrolisis, the thermal breaking of molecules into smaller ones, are eventually polar. This will explain the crescent rate of heating of the canola oil. From Hilder we get these results about water content in edible oil:
For testing, if this is caused by water or any other impurities, it would be interesting to pass the canola oil through some hydrophilic crystals and compare the results.
What is interesting for your experiments are the results of Hilder about the solubility of water in oil in function with temperature, which suggests that if you reduce the temperature of the oil to 0ºC for instance, you could separate the water by crystallization too:
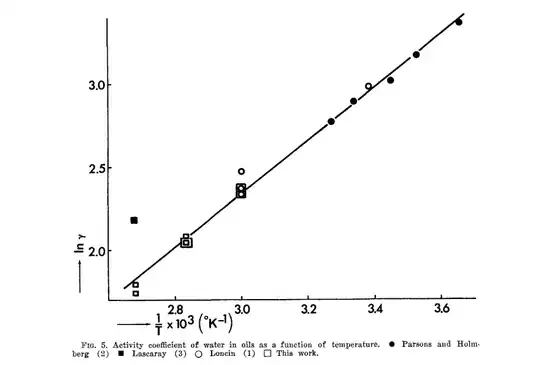
If your microwave contains just a cup of oil, with so little water, all microwave heating will be charged in this water component and this will increase considerably these water molecules, forcing a rapid degradation of the fatty acid molecules.
Suomelo (2017) study is particularly interesting for the verification of the relation between the heating and the degradation of the oil, showing that heating occurs together with the formation of (polar) peroxides. Actually, in the microwave oven this formation is even faster than in a conventional oven:
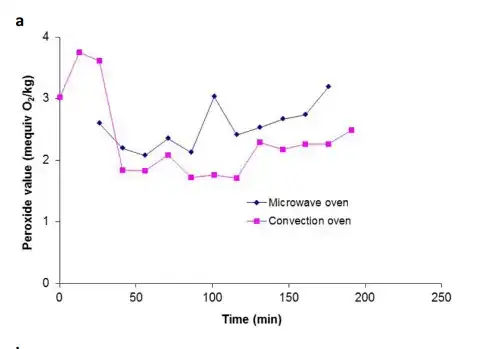
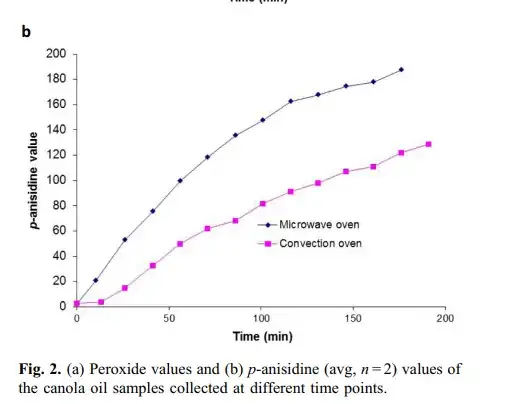
Could water solved in oil be the target of microwave heating?
Sure. Actually, Bo and Nyfers suggest this method as a way of detecting water dissolved in oils, important question in fuel industry.
The dielectric properties of a mixture of oil and water are determined by the permittivity and the relative volume fractions of each the constituents and of the structure of the mixing.
The Bruggeman formula is one such formula that is known to
represent real water/hydrocarbon mixtures with a high accuracy
$$
\frac{\epsilon_0 - \epsilon_{mix}}{\epsilon_0 - \epsilon_{w}} (\frac{\epsilon_{w}}{\epsilon_{mix}})^{\frac{1}{3}} = 1 - \Phi_0 = \Phi_w
$$
where $\epsilon_0$, $\epsilon_w$, $\epsilon_{mix}$ are the oil, water and mixture permittivities respectively, $\Phi_0$ is the relative oil volume fraction while $\Phi_0$ is the water volume fraction.
Degradation of Canola Oil in a microwave oven
After all, Canola Oil (or the components of its degradation) definitively heats up in a microwave oven:
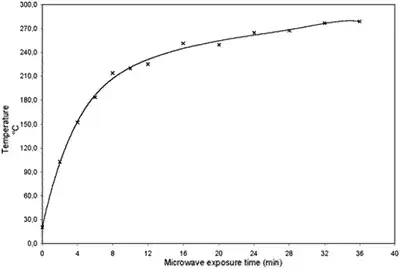
The heating of canola oil in a microwave involves several key processes due to its apolar nature and the unique way microwaves interact with different substances. Here are the primary points regarding the heating of canola oil as detailed in the paper:
Microwave Heating Mechanism:
- Unlike conventional heating, microwave heating occurs due to the interaction of electromagnetic fields with the molecules in the food, causing molecular friction and excitation.
- Foods with high moisture content typically heat faster due to their high dielectric constant and dielectric loss factors. However, apolar substances like canola oil have lower dielectric loss factors but still heat quickly due to their low specific heat .
Oxidative Stability and Degradation:
- The study found that microwave heating causes oxidative degradation in canola oil, as evidenced by the increase in absorptivity at 232 nm and 270 nm. These wavelengths correspond to the formation of conjugated dienes and trienes, respectively, which are products of lipid oxidation.
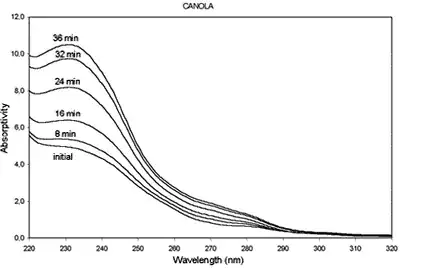
- Absorptivity at 232 nm (submitted to this frequency for spectrographic analysis) increased significantly after 12 minutes of microwave exposure, indicating an increase in oxidation products. Similarly, absorptivity at 270 nm, indicative of secondary oxidation products like unsaturated ketones and aldehydes, also increased .
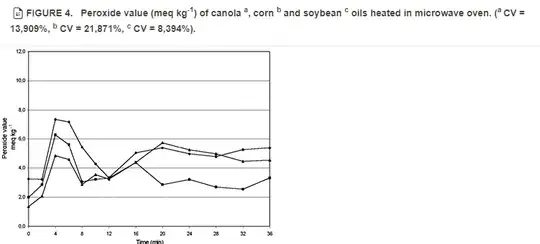
Peroxide and Acid Values:
- Peroxide value is a measure of primary oxidation products, specifically hydroperoxides. The peroxide value for canola oil initially increases during the first 6 minutes of microwave heating but then shows instability due to the decomposition of hydroperoxides at high temperatures.
- Acid value, which indicates the presence of free fatty acids resulting from hydrolytic degradation, also increases with microwave heating. This suggests that both oxidative and hydrolytic degradations occur during microwave heating .
UV-Spectrophotometric Analysis:
- UV-spectrophotometry is used to monitor the oxidative stability of canola oil by measuring absorptivity changes at specific wavelengths (232 nm and 270 nm). This method is effective in detecting both primary and secondary oxidation products and provides a clear indication of the extent of degradation .
Experimental Conditions and Observations:
- The study heated canola oil samples in a microwave oven (800 W, 2,450 MHz) for varying periods, up to 36 minutes. It was observed that temperature changes and the extent of degradation were similar across different vegetable oils (canola, corn, and soybean), highlighting that microwave-induced oxidation is a general phenomenon for these oils.
- It was also noted that the specific conditions of the microwave heating, such as power and exposure time, significantly affect the rate and extent of lipid oxidation .
That is consistent with the studies of Albi (1997) that provide a comprehensive comparison between microwave and conventional heating effects on the physical and chemical parameters of various edible oils and fats. To understand how these findings relate to the heating of canola oil specifically, we can draw some parallels and compare them to the results from the SciELO paper on microwave heating of canola oil.
Microwave Heating Mechanism:
- Both studies acknowledge that microwave heating involves the interaction of electromagnetic fields with the molecules in the substance being heated, causing molecular friction and excitation, which leads to heating. Albi et al. also mention that fats, including canola oil, have a great capacity for storing microwave energy despite their small dielectric loss factor.
Oxidative Stability and Degradation:
- The SciELO study reported increases in absorptivity at 232 nm and 270 nm for canola oil, indicating the formation of primary and secondary oxidation products. Albi et al. found similar results with other oils, showing significant increases in K232 and K270 values after microwave heating, which corresponds to the formation of peroxides, conjugated dienes, trienes, and other oxidation products.
- Albi et al. highlight that the internal friction of molecules during microwave heating contributes to greater oxidative degradation compared to conventional heating, consistent with the findings in the SciELO study.
Peroxide and Acid Values:
- In the Suomela study, the peroxide value initially increased but later became unstable due to the decomposition of hydroperoxides at high temperatures. Albi et al. do not specifically mention peroxide values but note that microwave heating led to a higher formation of oxidation products in general.
- Both studies observed increases in acid values with microwave heating, indicating hydrolytic degradation. This aligns with the formation of free fatty acids, a common indicator of oil degradation during heating.
Chemical and Physical Changes:
- Albi et al. measured various physical parameters, including density, viscosity, and refractive index, and found that microwave heating caused significant changes in these properties. For example, viscosity increased due to the formation of dimers and polymers, and density increased due to oxygen incorporation into oxidized triglycerides. These changes were more pronounced with microwave heating compared to conventional heating.
Comparison with Conventional Heating:
- Both studies found that microwave heating caused more severe degradation compared to conventional heating. Albi et al. noted that the effects of temperature and microwave energy combined resulted in greater changes in physical and chemical properties than conventional heating alone.
- The Suomela study similarly showed significant oxidative degradation of canola oil with microwave heating, aligning with Albi et al.'s findings that microwave heating can be more detrimental to the stability of edible oils.
Explanation of Canola Oil Heating in Microwave
Microwave Heating Mechanism:
Microwave heating is fundamentally different from conventional heating. In conventional heating, energy is transferred through convection, conduction, and radiation from the material's surface to its core. In contrast, microwave energy interacts directly with the material, converting electromagnetic energy into thermal energy through molecular interactions. This process involves several polarization mechanisms such as electronic, atomic, and dipole orientation polarizations.
Polar and Non-Polar Oils:
Polar molecules, such as water, interact strongly with microwave energy due to their permanent dipoles, leading to rapid heating. Non-polar oils, like canola oil, do not have permanent dipoles but can still absorb microwave energy through other mechanisms, such as ionic conduction and dielectric relaxation. Although the dielectric loss factor for non-polar oils is lower, they can still heat quickly because of their low specific heat capacity.
Oxidative Stability:
The Suomela paper highlights that microwave heating leads to oxidative degradation in canola oil, as evidenced by increased absorptivity at 232 nm and 270 nm, indicating the formation of conjugated dienes and trienes. The peroxide value, an indicator of primary oxidation products, shows significant alterations initially but becomes unstable at higher temperatures.
Polarization in Microwave Heating vs. Polar Characteristics of Molecules:
Polarization mechanisms in microwave heating involve the interaction of the electromagnetic field with molecular dipoles, free ions, and interfacial charges. This is different from the intrinsic polar characteristics of molecules. Even non-polar molecules like those in canola oil can experience heating due to the microwave field's ability to induce polarization effects through mechanisms such as ionic conduction and dielectric relaxation.
Conclusion:
Microwave heating of canola oil leads to significant oxidative degradation, forming primary and secondary oxidation products. While non-polar oils like canola oil have lower dielectric loss factors, they can still heat efficiently in a microwave due to low specific heat capacity and other polarization mechanisms.
For a more detailed understanding of these processes and their effects on canola oil, further examination of the specific methods and findings in bibliography is recommended.
Bibliography
Albi, T., Lanzón, A., Guinda, A., Pérez-Camino, M. C., & León, M. (1997). Microwave and Conventional Heating Effects on Some Physical and Chemical Parameters of Edible Fats. Journal of Agricultural and Food Chemistry, 45(8), 3000–3003. doi:10.1021/jf970168c
Angela, A., & dAmore, M. (2012). Relevance of Dielectric Properties in Microwave Assisted Processes. InTech. doi: 10.5772/51098
Lund Bo, O., & Nyfors, E. (2002). Application of microwave spectroscopy for the detection of water fraction and water salinity in water/oil/gas pipe flow. Journal of Non-Crystalline Solids, 305(1-3), 345–353. doi:10.1016/s0022-3093(02)01130-4
Hilder, M. H. (1968). The solubility of water in edible oils and fats. Journal of the American Oil Chemists’ Society, 45(10), 703–707. doi:10.1007/bf02541262
Suomela, J.-P., Tarvainen, M., & Kallio, H. (2017). Effects of microwave vs. convection oven heating on the formation of oxidation products in canola (Brassica rapa subsp. oleifera) oil. OCL, 24(3), A301. doi:10.1051/ocl/2017015
VIEIRA, T., REGITANO-D'ARCE, M., & , (1998). STABILITY OF OILS HEATED BY MICROWAVE: UV - SPECTROPHOTOMETRIC EVALUATION. Ciência e Tecnologia de Alimentos,






