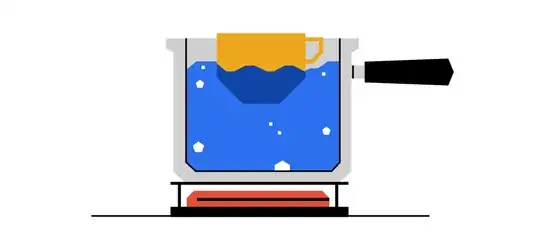
The question asked by a website is as such (possibly behind a paywall):
A cup of warm water is suspended in a large pot of water held at a steady boil. Will the water in the cup ever boil? Assume that the pot never runs out of water.
The provided answer & explanation:
No. When the cup of water is placed in the boiling water, the cup is cooler than the surrounding water and heat will flow into it. Eventually, the water in the cup will increase to $100 ^{\,\circ} $ C — if any more energy goes into the cup, then the cup will begin boiling. But at this point, the boiling water in the pot and the $100 ^{\,\circ}$ C water in the cup are at the same temperature. Since there is no temperature difference, there will be no more heat flow into the cup, so it will never boil.
According to the explanation (emboldened sentence), the water in the cup does reach 100 degrees. Doesn't that mean that the water in the cup does indeed boil, because the boiling point of water happens to be at 100 degrees Celsius? Additionally, I don't really get the difference (temp or state wise) between the cup-water & the water in the pot, because the heat source is the same and it ought to always flow into the cup to reach thermal equilibrium?