I’ve been reading about the thermoelectric effect and Peltier cells, but one thing the sources seem to gloss over is where the cooling or heating actually occurs. They all seem to say “at the junction of the two materials”. What I’m wondering is, if one where to prevent any heat transfer between the materials (say an infinitely thin, infinitely electrically conductive, perfect thermal insulator placed in-between), where would heating/cooling occur?
2 Answers
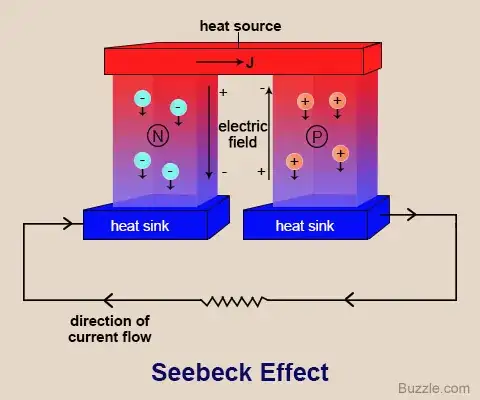 Taken from: https://sciencestruck.com/seebeck-effect-with-applications
Taken from: https://sciencestruck.com/seebeck-effect-with-applications
In the diagram above, you can see that heat does not actually travel between junction materials. Heat does not flow from junction material A to junction material B resulting in A being colder than B.
Instead, each individual junction material sits in a temperature gradient. In other words, A has a different temperature on each end, and B also has a different temperature on each end. It's not the temperature difference between junction materials that matters. It's the temperature difference within the same junction material.
So that was the Seebeck effect where you apply a temperature gradient to produce current. The Peltier effect is just in reverse. So you apply current to produce a temperature gradient.
Therefore, since your electrically conductive, thermally conductive material still allows current to flow between junction materials, and since the temperature gradient is within each junction metal rather than between junction metals, the thermal insulation does nothing and the system still works like normal.
- 9,389
Your title question is broader and has a different answer than what you ask in the body of your text.
Regarding the body of your text:
In a Peltier cell, the n and p materials make contact usually with copper or gold. In that case, n and p materials (semiconductors) have "high" Seebeck coefficient, of the order of $400$ µV/K, while metals (copper/gold) have at least an order of magnitude smaller Seebeck coefficient. What happens at the interface metal/semiconductor, is a heat generated, proportional to the difference in the Seebeck coefficients of the two interfaced materials. This heat is called the Peltier heat, and has a magnitude worth $(S_\text{A}-S_\text{B})TI$. This heat is generated right at the interface, it is not a volume-generated heat. The only ways to prevent this Peltier heat to generate is to either remove the current ($I=0$), pick two different materials which happen to possess the same Seebeck coefficient, or reach absolute zero temperature (of course impossible in practice, unlike the previous two).
Regarding thermoelectricity in general, to be short, there are several different possible volume-heat generations (Thomson and Bridgman heats to name the two most famous), these heats occur at every single point inside the thermoelectric materials. Some of them require a thermal gradient to exist, others require an anisotropy in the Seebeck coefficient (amongst other prerequisites), some of them require a position-dependent doping to occur, etc.