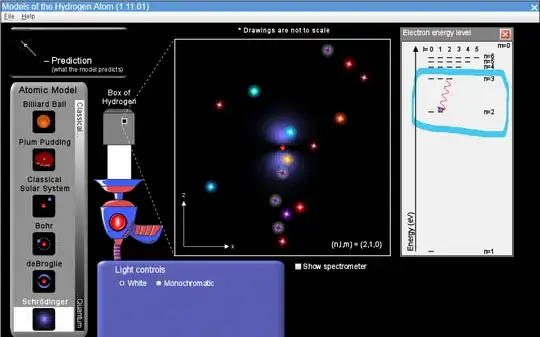
Rydberg's formula can be used to find the wavelength emitted due to an electron moving from one energy level $n_i$ to another one $n_f$. But when the Schrödinger's model of the atom is introduced we learn that there is more than one quantum number, known as $l$ and $m$. How can one calculate the wavelength of a photon emitted taking into account, for example, the quantum number $l$? Is there any formula?
For example, in the image I attached why is it that when the electron moves from the level 3d to the level 2p the light emitted is red? I mean is it always the case that the emitted light only depends on the quantum number n? and well one would have to add the condition $\Delta l=l_{i}-l_{f}=\pm1$
Sorry if is some sort of obvious question but I couldn't find any information (or didn't search well enough) that would help me confirm this assumption. Thanks in advance.