I would like to address something the other answers do not mention, that is, what really is happening at the quantum level when we say "the banana is reflecting". When photons (I am only talking about visible wavelength light) are incident on the banana, two things can happen:
absorption and re-emission. Most of the photons that get absorbed by the banana, will either heat up the banana peel (transfer their kinetic energies to the molecules vibrational, rotational, and translational energies), or will be absorbed and re-emitted (through electronic transitions), and these photons will be part of the emission spectrum we can see in the other answers. Please note that even in this case, the angle of these photons is random, regardless of the incident angle. It is very important to understand that in this case the photons cease to exist, and new photons are re-emitted.
diffuse reflection.
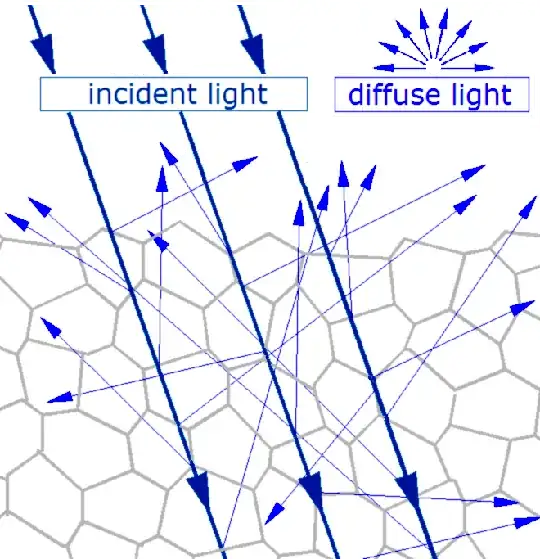
https://en.wikipedia.org/wiki/Diffuse_reflection
These photons will be another part of the emission spectrum that you can see in the other answers. Please note that the angle of these photons will be random, regardless of the incident angle. It is very important to understand that in this case, the photons do not cease to exist, the same photon is reflected.
Please note that in both cases, the angle of the photons (including the ones that reach our eyes and create the picture and colors of the banana) will be random, regardless of the incident angle, this is part of the reasons (and flatness of the surface at the atomic level) why bananas are usually not shiny.
Bottom line, whatever picture we have of the banana, it is because of these two ways of photons (diffuse reflection, absorption and re-emission) that come into our eyes that create this image in our brain, and these are made up of all kinds of wavelengths (as you can see from the other answers), that combine into the perception of the yellow or green banana, in our brain (and the appearance of a usually non shiny surface).
I just wanted to clarify that the answer to your question includes this explanation when we use the words "banana is reflecting".



