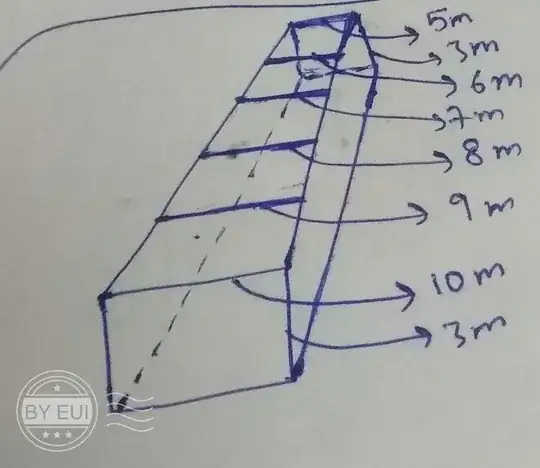
This question is quite long (and stupid) and needs focus and patience while reading since there are seriously a lot of ideal assumptions. So please tolerate if possible.
So suppose we a have a tube of rectangular cross section (as shown in the fig.).
Now let's assume that at t=0 we have a plane of molecules just entering the tube from the side with wider hole such that the speed of all the molecules in this plane is same (let's assume if not possible).
Here comes another assumption :
suppose the molecules in the entering plane are arranged in such an ordered manner and that the theoretical minimum possible distance between two adjacent molecules is $1 m$ (with respect to the units in the fig) and in this plane there are overall 30 molecules (this means that the molecules can't be further compressed) .
Now as they move forward and reach the second cross section (of area $27$$m^2$) the number of molecules in the plane must go down to 27. This means that out of 30 molecules only 27 could have the same speed variation as they moved from first cross section to the second one. Similarly if we continue this argument this will mean that after reaching the third cross section there will be only $24$ molecule sin the plane and so on.
This means that as we move ahead in the tube the number of molecules in the plane goes on decreasing. One can ask where are the other $3$ molecules going after each cross section is being crossed?*
So I would say it directly that "the $3$ molecules leaving the plane each time are having a greater change in speed than the majority of the molecules in their plane" .
I am making this claim on the basis of the fact that (number of molecules entering the tube must be equal to the number of molecules leaving it). So for $30$ molecules to leave , we must have $15$ such speeded molecules too so that they can join the other $15$ molecules in the plane of the last cross section.
But this comes down to another question (about which I don't know the answer) : What makes those $3$ molecules so special ? Why did they experience greater change in speed than the majority in their plane and how did this happen ?