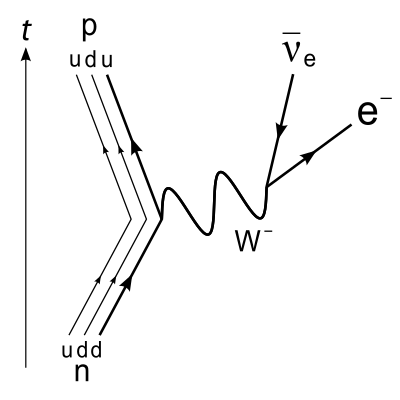
Decay of proton to neutron is--
- Possible only inside nucleus
2)Not possible
- Always possible as it is associated with Beta+ decay
I thought that mass should be conserved here, but we know that neutron mass is more than proton,so how in any emission can proton decay into a neutron? [The correct answer given here is 2,i.e(possible only inside nucleus)]
One analogy that i came with is maybe, according to Einstein's equation, we should give some energy to make this decay happen, so that the extra mass is provided by energy, according to E=mc^2.
Is that the correct analogy? I'm still not convinced that decay of proton to neutron should happen,can someone please explain it in detail.