https://en.wikipedia.org/wiki/Enthalpy#Definintion
here must What does "work required to achieve its pressure and volume:" mean?
This is what I did make out of given answers :
$$dQ=dU+PdV$$
for this work done by system is PdV
now meaning considering total work to form is = internal energy + work done to occupy the space which is
$$nadT=d(PV)$$
therefore this total is going to be really:
$$dH=dU+d(PV)$$
$$dH=dQ+dP**V-dW(shaft \ work)$$
for opposite sign convention -dPV.
3 Answers
The article is trying to provide a physical interpretation to the enthalpy. All this ever does is confuse the student. Enthalpy is not a fundamental quantity like internal energy or entropy. It is just a convenient function to work with in thermodynamics so that you don't have to write U+PV all the time.
- 35,124
What does "work required to achieve its pressure and volume:" mean?
As @Chet Miller pointed out, enthalpy is not a fundamental property like internal energy, entropy, pressure, volume, and temperature. I like to call it a derived property because it is derived from other fundamental properties, in this case, internal energy pressure and volume. Other derived properties are the Gibbs Free Energy and Helmholtz Free Energy.
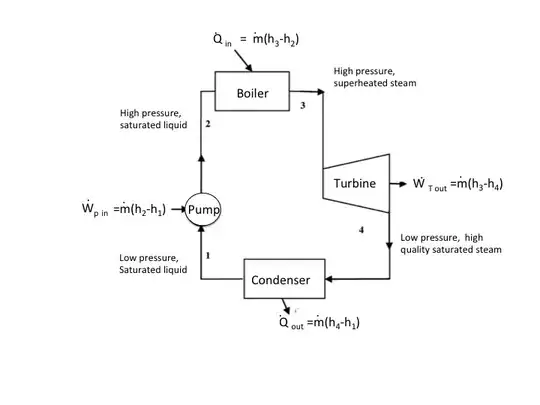
That being said, it is an extremely useful tool for analyzing many open systems. The Wiki statement on enthalpy being the "work required to achieve its pressure and volume" can be illustrated by the Rankine steam cycle. See the diagram below.
The cycle consists of four major components (control volumes): Turbine, Boiler, Condenser, and pump (or compressor). The work associated with the pump and turbine, and the heat transfers associated with the boiler and condenser, can all be expressed in terms of the change in enthalpy of the working fluid (water and/or steam) between the inlet and outlet of each control volume of the cycle. The enthalpies changes include both the changes in internal energy as well as the product of pressure and volume, $\Delta h=\Delta u+\Delta (pV)$, as applicable.
Consider, for example, the pump. The input to the pump (1) is low pressure (generally atmospheric) saturated liquid water that leaves the condenser. To move this low-pressure liquid into the high-pressure boiler (2), the pump needs to increase the pressure of the liquid output of the condenser to force it into the boiler, which then heats the liquid converting it to steam. Since liquid water is relatively incompressible, the work done by the pump consists of constant volume flow work or $Vdp$ work.
Hope this helps.
- 81,786
To add a bit to the earlier answers regarding enthalpy.
Enthalpy accounts for the energy associated with mass flow in/out of an open thermodynamic system.
The specific enthalpy $h$ (enthalpy per unit mass) is $h = u + pv$ where $u$ is specific internal energy, $p$ is pressure, and $v$ is specific volume. In the energy balance for the open system, the energy added to/removed form the system by mass flow is accounted for considering the enthalpy in/out of the system. The $pv$ term is called flow energy from an Eulerian viewpoint-fixed in space- as is used for an open thermodynamic system. (From a Lagrangian viewpoint- following a fixed mass- $pv$ is called flow work.)
In general the specific energy associated with mass flow is $h + V^2/2 + gZ$ where $V$ is velocity $g$ is the acceleration of gravity, and $Z$ is elevation. This accounts the kinetic and potential energy per unit mass for mass flowing in/out of an open thermodynamic system in in addition to the enthalpy.
For a closed thermodynamic system (no mass flow in/out) enthalpy is associated with a constant pressure process. For a closed system $Q - W = \Delta U$ where $Q$ is heat added to the system, $W$ is work done by the system, and $\Delta U$ is change in internal energy, $U$, of the system. For the case where heat is slowly added at constant pressure, the work done by the system is $p \Delta $V and for constant pressure this is $\Delta (p$V$)$. Therefore, $Q = \Delta H$. $H$ is the enthalpy of the system equal to $U + p$V where , $p$ is pressure, and V is volume. $\Delta H$ is the change in the enthalpy of the closed system.
I suggest you consult a good text on Thermodynamics, such as one by Sonntag and Van Wylen.
- 9,601