I don't have a definite answer for you. But I don't believe the existing answers have given this an appropriate treatment and I would like to leave an extended comment.
You can extract useful work from a temperature gradient-- e.g. a different in temperature. Many thermodynamic processes will only care about the difference in temperature rather than absolute values (e.g. Heat Equation is invariant, still satisfied, under the addition of a constant term to temperature). Energy is by its very definition a numerical quantity which is conserved due to the time-invariance of laws of physics. It so happens that something which is 'hot' has a high amount of this quantity, and something which is 'cold' has a small amount.
The article you linked says:
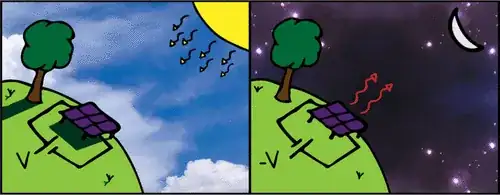
...a sky-facing surface passes its heat to the atmosphere as thermal radiation, losing some of its heat to space and reaching a cooler temperature than the surrounding air.
What this means is that the device creates a temperature difference between itself and the ambient air. That temperature difference can then be harnessed to do 'useful work' (e.g. charge an electrical battery). What's happening is that the device is radiating some of Earth's heat energy to space, and heat from the ambient air must flow in to replace it. That heat flow comes from a difference in temperature between the device and the ambient air. That is where the 'useful work' is coming from. What happens is that some energy is taken from the higher-temperature heat source (ambient air) and dumped into the lower-temperature one (the device), and some of it goes toward a practical purpose like charging a battery.
In regards to your question:
We know that the heat can be converted into heat energy with the help of thermoelectric generators but why can't we generate energy from coldness. Like the temperature of the universe in 1K, can this be used in near future to be used as an energy resource for probes or satellites?
At first I was going to say a tentative no. However, thinknig more about it I suppose it is possible in principle to radiate the ambient 'heat' of the probe away faster than it is absorbed from space. This could be done if the probe has a surface highly reflective to infrared light, but still acts effectively as a heat radiator. So this may be an engineering problem rather than a physical impossibility. With clever optics perhaps it is even possible to focus and trap heat in an optical well, so that a higher temperature (I don't know, maybe 10K) is achieved from which a heat difference (10K versus 1K of ambient space) can be used to extract useful work. I do not know if this is possible but I don't see any obvious physical principle preventing this.
I hope an expert can elaborate on this.