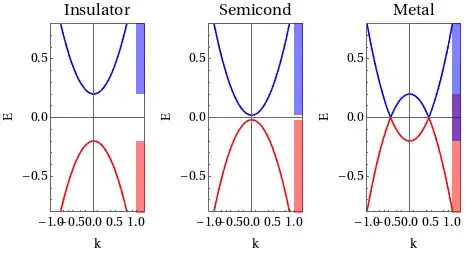
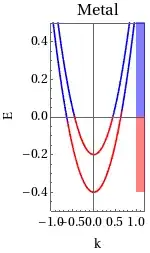
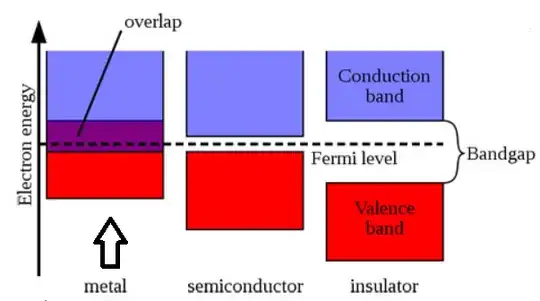
 As per Energy Band Diagram of metals, the conduction band and valence bands overlap, But if we assume there is overlapping then the total number of energy bands will reduce as hence electron will have less number of energy states to occupy, which will violate Pauli exclusion principle.
As per Energy Band Diagram of metals, the conduction band and valence bands overlap, But if we assume there is overlapping then the total number of energy bands will reduce as hence electron will have less number of energy states to occupy, which will violate Pauli exclusion principle.
For Example, suppose, if there are N states in Valance band and M states in Conduction band and total M+N electrons. Assume 10% of overlapping of states, we will then have 0.9M+N states remaining, but since the total electron is still M+N even after overlapping, Electrons will have to occupy the same energy state i.e. more than 1 electron will be occupied in the same energy state, But this is not allowed by Pauli exclusion principle...
My question is do overlapping of energy band is allowed? If allowed then where I am Wrong?