The interaction time between light and electron is extremely fast $\sim10^{−15}$
seconds. So in that sense electrons are excellent time keepers. For two photons to interact with the same electron, they’d have to arrive within $10^{-15}$ seconds of each other. The probability of this is still not zero. These events do happen. But single photon interactions happen way way way more often. So they overshadow these events.
One way to observe two photon absorption is to send high intensity light of photon energy that is lower than the threshold energy. This way, single photon interactions don’t lead to ejection of electron. And the only way any electron will be ejected is via multiple photon interaction.
One last attempt
I’ll try one last time to explain why two photon interactions are very rare without special setups. Fundamentally, it is a interesting question.
I think the source of your confusion is your mental picture of light. You might know that light is made up of photons. Now there can be different kinds of light. Light from lasers, light from bulb and so on. The main distinguishing feature between these different kinds of light is how the photons are distributed in a given time window. Here’s a cartoon depicting some of them:
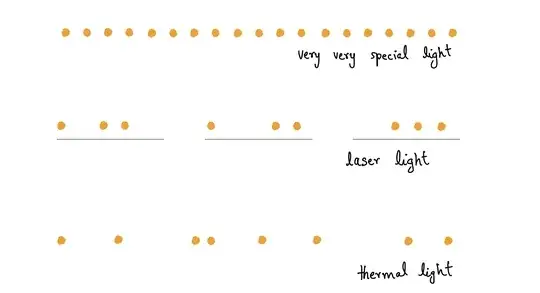
As you can see, for the most common type of light (from bulb and so on), thermal light is made up of photons that are randomly distributed. Lasers on the other hand are still randomly distributed, but average number of photons in a subframe is constant (three in the cartoon). What you might be thinking of as the distribution (equally spaced photons) is actually an extremely rare one called number state. These are generated under very special circumstances.
Coming to the photoelectric effect, consider a stream of photons coming towards a metal sheet. The electrons interact within a small time frame depicted by a blue box in the following cartoon.

You can see that it’s very rare to have two photons inside the box simultaneously. And whatever electrons you’ll be getting will be mostly because of single photon excitation.
However, if we increase the intensity (number of photons in a time window) much higher then the probability of two photons coming within the blue box is higher.

Now if you want to detect these two-photon excitation, then you’ll have to filter out single photon excitations. One easy way to do that is to choose photons such that individually they don’t have enough energy to excite the electron. But two together they do.