Why do electrons form shells/clouds around nucleus? If electron is negative charge and protons in nucleus are positive they should attract each other and as a end result stick in one place?
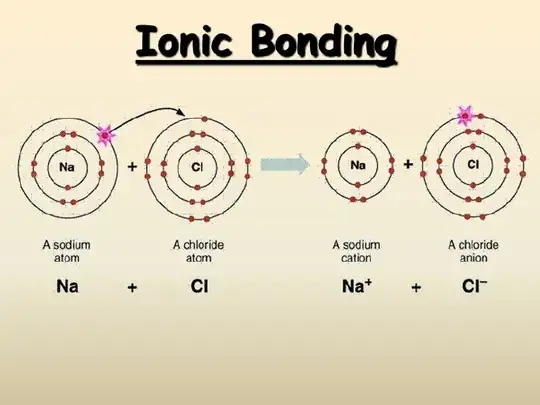
Second question (an I think it's related) is about ionic bonding. I think this is most popular example:
But why does the electron jump from Na to CL? The octet rule makes very less sense for me, as it says "Atoms try to get 8 electron in outer shell", and it's rather incomplete and abstract explanation.
And more over, why is is sometimes Ionic Bonding and sometimes Covalent Bonding?
Edit:
Dear members stop voting for duplicate and read the whole thing. This topic answers multiple linked questions, not just one (1st that is similar) and I picked already a solution.