I have read these threads
If black is the best absorber and radiator, why does it get hot?
Black and white matters. But why and how?
If a black body is a perfect absorber, why does it emit anything?
Why is black the best emitter?
Some respondents referred to the Stefan-Boltzmann Law and indeed were kind enough to do the calculation. This post
Emissivity and Final Temperature of a Black and White object
indicates that the emissivity constant should be different for white objects than for black objects. Wikipedia shows for example
https://en.wikipedia.org/wiki/Emissivity
states that 'white paint absorbs very little visible light. However, at an infrared wavelength of 10x10−6 metres, paint absorbs light very well, and has a high emissivity. '
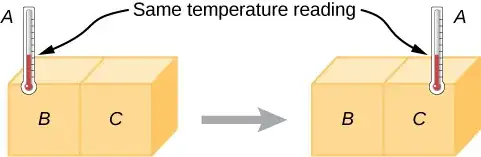
I am still at a loss though as to how to apply the Stefan-Boltzmann equation to calculate the equilibrium temperature of two identical objects (for example a piece of paper) in the identical sunlight(light intensity of 1000 W/m2 (typical for cloudless sunny day)) that differ only in color.