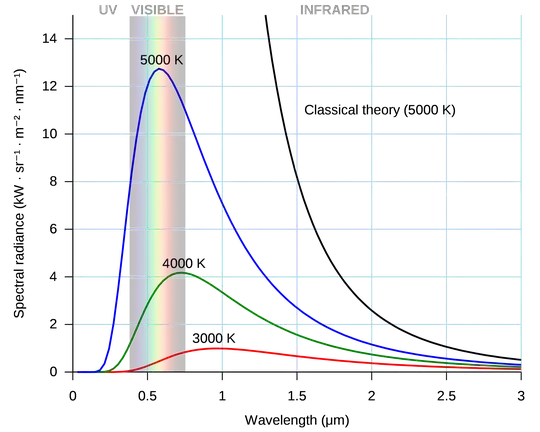
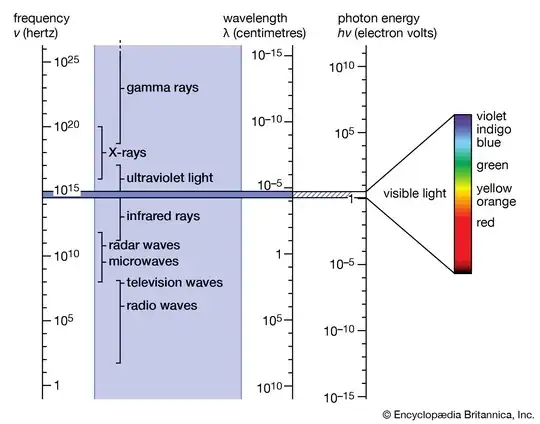
I have a very rudimentary understanding of electromagnetic radiation and how it corresponds to temperature.
It is my understanding that any object above absolute zero first starts emitting radiation in infrared, and then as its temperature increases, it starts emitting in visible light from red to orange to white to blue and so on.
But why does it start at infrared? Why not start at radio waves or microwaves first?