Here is nucleus binding energy chart :
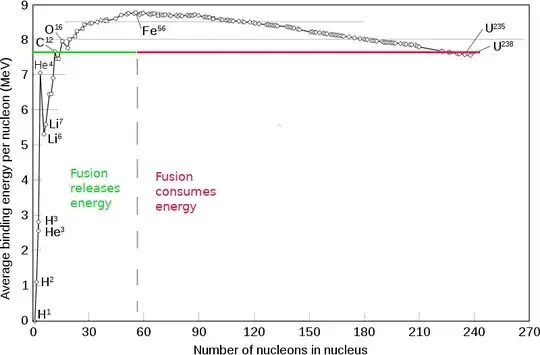
There's so called "Iron peak" as you see. Element fusion until $\text{Fe}$ - produces energy, fusion of iron and heavier elements- consumes energy from surroundings, in the form of heat, kinetic energy of neutrons, etc, so they can be going on only in extreme conditions, such as supernovas. As about your question, here is one iron production scheme :
$$
4 ({}^1H) \to {}^4He + 2 e^+ + 2 n + \gamma
\\3 ({}^4He) \to {}^{12}C + \gamma
\\{}^{12}C + {}^{12}C \to {}^{24}Mg + \gamma
\\{}^{12}C + {}^{4} He \to {}^{16}O + \gamma
\\{}^{16}O + {}^{16}O \to {}^{32}S + \gamma
\\{}^{16}O + {}^{4} He \to {}^{20}Ne + \gamma
\\{}^{28}Si + 7({}^{4} He) \to {}^{56}Ni + \gamma
\\{}^{56}Ni \to {}^{56}Co + e^+
\\{}^{56}Co \to {}^{56}Fe + e^+
$$
Last pair of reactions are beta decay of unstable Nickel and Cobalt isotopes. But this chain reaction requires a high-mass star. So iron in abundant amount levels is produced in other massive stars or supernova explosions.
